1056039-83-8
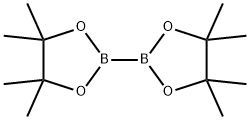
 1056039-83-8 结构式
1056039-83-8 结构式
基本信息
泰地唑胺中间体 H
磷酸特地唑胺中间体4
特地唑胺中间体TD-1.1
磷酸特地唑胺中间体4 1KG
2-(2-甲基-2H-四唑-5-基)-5-硼酸频哪醇酯吡啶
2-(2-甲基-2H-四唑-5-基)吡啶-5-硼酸频哪醇酯
2-(2-甲基-2H-四唑-5-基)-5-(4,4,5,5-四甲基-1,3-二氧硼烷-2-基)吡啶
2-(2-甲基-2H-四唑-5-基)-5-(4,4,5,5-四甲基-1,3,2-二噁硼烷-2-基)吡啶
2-(2-甲基-2H-四唑-5-基)-5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡啶
Tedizolid intermediate H
2-(2-Methyl-2H-tetrazol-5-yl)-5-(4
Pinacol 2-(2-methyl-2H-tetrazol-5-yl)-5-pyridineboronate
2-(2-Methyl-2H-tetrazol-5-yl)pyridine-5-boronic acid pinacol
2-(2-Methyl-2H-tetrazol-5-yl)pyridine-5-boronic acid pinacol ester
2-(2-Methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)pyridine
Pyridine, 2-(2-methyl-2H-tetrazol-5-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
物理化学性质
制备方法
73183-34-3

380380-64-3

1056039-83-8
向2-(2-甲基-四氮唑)-5-溴吡啶(1.00g,4.17mmol)在干燥脱气的1,4-二恶烷(15mL)中的溶液中加入双(频哪醇合)二硼(1.27g,5.00mmol)、乙酸钾(1.35g,13.75mmol)和Pd(dppf)Cl2(0.26g,0.35mmol)。在氩气保护下,将反应混合物置于密封管中,于80℃加热搅拌3小时。反应完成后,将混合物通过硅胶柱快速过滤,用二氯甲烷作为洗脱剂。收集有机层,减压浓缩,残余物用正己烷洗涤,得到白色固体产物2-(2-甲基-2H-四唑-5-基)-5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)吡啶(1.03g,收率86.3%)。产物经1H-NMR(400MHz,CDCl3)表征:δ 9.14(s,1H),8.30(m,2H),4.48(s,3H),1.38(s,12H)。
参考文献:
[1] Chemical and Pharmaceutical Bulletin, 2015, vol. 63, # 2, p. 143 - 146
[2] Patent: WO2008/108988, 2008, A1. Location in patent: Page/Page column 53
[3] European Journal of Organic Chemistry, 2016, vol. 2016, # 7, p. 1305 - 1313
[4] Patent: WO2009/74812, 2009, A1. Location in patent: Page/Page column 41
[5] Patent: US2010/69441, 2010, A1. Location in patent: Page/Page column 21-22

