1121057-77-9
 1121057-77-9 结构式
1121057-77-9 结构式
基本信息
N-叔丁氧碳酰基-3,4-二氢吡啶-5-硼酸酯
5-硼酸频哪醇酯基-3,4-二氢吡啶-1(2H)-羧酸叔丁酯
5-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)-3,4-二氢吡啶-1(2H)-羧酸叔丁酯
tert-Butyl 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dih
tert-butyl 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1
(1-(TERT-BUTOXYCARBONYL)-1,4,5,6-TETRAHYDROPYRIDIN-3-YL)BORONIC ACID PINACOL ESTER
tert-butyl 5-(tetramethyl-1,3,2-dioxaborolan-2-yl)-1,2,3,4-tetrahydropyridine-1-carboxylate
tert-Butyl 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,4-dihydropyridine-1(2H)-carboxyla
1(2H)-Pyridinecarboxylic acid, 3,4-dihydro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
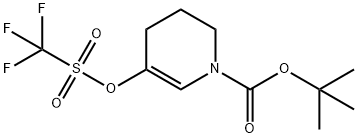
149108-74-7
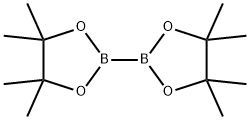
73183-34-3

1121057-77-9
步骤4. 将5-(((三氟甲基)磺酰基)氧基)-3,4-二氢吡啶-1(2H)-羧酸叔丁酯(1.45g,4.38mmol)和联硼酸频那醇酯(1.223g,4.81mmol)溶于二恶烷(24mL)中。向此溶液中加入乙酸钾(1.289g,13.13mmol)和PdCl2(dppf)-CH2Cl2加合物(0.107g,0.131mmol)。在氮气保护下,将反应混合物吹扫5分钟,随后在80℃油浴中加热过夜。反应完成后,将混合物在乙酸乙酯和水之间分配。分离有机层,用盐水洗涤,经无水硫酸钠干燥,过滤并浓缩。通过快速色谱法纯化残余物,使用0-30%乙酸乙酯/庚烷梯度洗脱,得到目标产物5-硼酸频哪醇酯基-3,4-二氢吡啶-1(2H)-羧酸叔丁酯,为高粘度液体(1.2g,收率89%)。LCMS(m/z):254.1(MH+-tBu),保留时间1.21分钟。
参考文献:
[1] Patent: US9242996, 2016, B2. Location in patent: Page/Page column 385; 386; 388; 389
[2] Patent: WO2018/203256, 2018, A1. Location in patent: Page/Page column 36
[3] Patent: US2013/102601, 2013, A1. Location in patent: Paragraph 184
[4] Patent: US2013/102600, 2013, A1. Location in patent: Page/Page column 0169; 0171
[5] Patent: WO2015/48662, 2015, A2. Location in patent: Page/Page column 44-45
