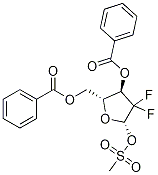
122111-11-9
 122111-11-9 结构式
122111-11-9 结构式
基本信息
D-ERYTHRO-PENTOFURANOSE, 2-DEOXY-2,2-DIFLUORO-, 3,5-DIBENZOATE 1-METHANESULFONATE
2-DEXY-2,2-DIFLUORO-3,5-O-DIBENZOYLRIBOSE MESYLATE
2-Deoxy-2,2-difluoro-D-erythro-ribofuranose-3,5-dibenzoate 1-Methanesulfonate
物理化学性质
制备方法
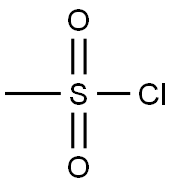
124-63-0

1173824-58-2
134877-42-2
将((2R,3R)-3-(苯甲酰氧基)-4,4-二氟-5-羟基四氢呋喃-2-基)甲基苯甲酸酯(4.14 g,10.9 mmol)溶解于无水二氯甲烷(52 mL)中,加入无水三乙胺(2.4 mL),将溶液冷却至0℃。在搅拌下缓慢滴加甲基磺酰氯(1.23 mL,15.8 mmol)。反应混合物在室温下搅拌18小时。反应完成后,将混合物用二氯甲烷(140 mL)稀释,并用饱和碳酸氢钠溶液(56 mL)洗涤。有机相用无水硫酸钠干燥,减压浓缩,得到油状产物,为异构体混合物(5.03 g,定量收率)。 19F NMR(CDCl3, 471 MHz):δ -107.70, -108.22, -120.65, -121.17, -122.21, -122.73, -123.76, -124.45。 主要异构体(60%)的1H NMR(CDCl3, 500 MHz):δ 8.13-8.04(m, 4H, Bz), 7.65-7.54(m, 2H, Bz), 7.50-7.41(m, 4H, Bz), 6.17(d, J = 5.6 Hz, 1H, H-1), 5.62(dd, J1 = 4.2 Hz, J2 = 16.4 Hz, 1H, H-3), 4.91(q, J = 3.9 Hz, 1H, H-4), 4.81-4.61(m, 2H, H-5), 3.17(s, 3H, CH3)。 次要异构体(40%)的1H NMR(CDCl3, 500 MHz):δ 8.13-8.04(m, 4H, Bz), 7.65-7.54(m, 2H, Bz), 7.50-7.41(m, 4H, Bz), 6.09(d, J = 6.4 Hz, 1H, H-1), 5.98(dt, J1 = 7.3 Hz, J2 = 15.0 Hz, 1H, H-3), 4.81-4.61(m, 3H, H-4, H-5), 3.03(s, 3H, CH3)。 13C NMR(CDCl3, 126 MHz):δ 40.09, 40.20(CH3), 62.52, 63.08(C-5), 69.61(dd, J1C-F = 15.7 Hz, J2C-F = 26.0 Hz, C-3), 71.04(dd, J1C-F = 17.4 Hz, J2C-F = 36.4 Hz, C-3), 79.68, 79.75, 82.59(C-4), 98.81(dd, J1C-F = 25.0 Hz, J2C-F = 41.8 Hz, C-1), 99.52(dd, J1C-F = 24.5 Hz, J2C-F = 46.3 Hz, C-1), 120.61(dd, J1C-F = 253.5 Hz, J2C-F = 269.8 Hz, C-2), 120.91(dd, J1C-F = 249.3 Hz, J2C-F = 276.3 Hz, C-2), 128.42, 128.58, 128.63, 128.70, 128.76, 128.79(Ph), 129.18, 129.25('ipso' Ph), 129.76, 130.07, 130.14, 133.51, 133.63, 134.19, 134.26(Ph), 164.89, 165.03, 165.81, 165.90(CO)。
参考文献:
[1] Synthesis, 1992, # 6, p. 565 - 570
[2] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 14, p. 4338 - 4345
[3] Patent: WO2009/61781, 2009, A1. Location in patent: Page/Page column 102-103
[4] Biochemistry, 2010, vol. 49, # 7, p. 1404 - 1417
[5] Patent: WO2005/95430, 2005, A1. Location in patent: Page/Page column 18
