123986-64-1
 123986-64-1 结构式
123986-64-1 结构式
基本信息
N-BOC-4-(氨甲基)苯甲醇
4-(羟甲基)苄基氨基甲酸叔丁酯
4-(BOC-氨基甲基-苯基)-甲醇
(4-羟基甲基苄基)-氨基甲酸叔丁酯
4-(N-Boc-aMinoMethyl)benzyl alcohol
N-Boc-4-(aminomethyl)benzyl Alcohol
tert-BUTYL-4-(HYDROXYMETHYL)BENZYLCARBAMATE
4-(Aminomethyl)benzyl alcohol, N-BOC protected
4-(Aminomethyl)benzylalcohol,N-BOCprotected97%
4-(tert-Butoxycarbonylaminomethyl)benzyl alcohol
tert-Butyl [4-(hydroxymethyl)benzyl]carbamate 97%
4-(N-TERT-BUTOXYCARBONYLAMINOMETHYL)BENZYL ALCOHOL
4-(Aminomethyl)benzyl alcohol, N-BOC protected 97%
物理化学性质
制备方法
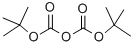
24424-99-5

39895-56-2

123986-64-1
一般步骤:将4-(氨基甲基)苯甲醇(1 g,7.29 mmol)与二碳酸二叔丁酯(1.59 g,7.29 mmol)在二氯甲烷(30 mL)中的混合物于室温下搅拌反应过夜。反应完成后,将反应混合物在减压下浓缩以除去溶剂。粗产物通过硅胶柱色谱法纯化,洗脱剂为二氯甲烷与乙酸乙酯的混合溶剂(体积比1:1),得到4-(羟甲基)苄基氨基甲酸叔丁酯(0.8 g,2.28 mmol,收率31%)为白色固体。产物经1H NMR(400 MHz,DMSO-d6)表征:δ 7.37(s,1H),7.22(dd,J = 27.8, 8.0 Hz,4H),5.13(t,J = 5.7 Hz,1H),4.46(d,J = 5.7 Hz,2H),4.10(d,J = 6.1 Hz,2H),1.36(s,9H)。
参考文献:
[1] Patent: WO2006/12135, 2006, A1. Location in patent: Page/Page column 49-51
[2] Patent: US2006/293379, 2006, A1. Location in patent: Page/Page column 63
[3] Angewandte Chemie - International Edition, 2012, vol. 51, # 4, p. 941 - 944
[4] Chemical Communications, 2010, vol. 46, # 7, p. 1073 - 1075
[5] Journal of Medicinal Chemistry, 2009, vol. 52, # 1, p. 33 - 47
