131001-86-0
 131001-86-0 结构式
131001-86-0 结构式
基本信息
2-溴-3-甲基苯甲酸甲酯
甲基 2-溴-3-甲基苯甲酸酯
2-溴-3-甲基苯甲酸甲酯 50G
METHYL 2-BROMO-3-METHYLBENZOATE
Methyl 3-Methyl-2-Bromobenzoate
2-Bromo-m-toluic acid methyl ester
Methyl 2-Bromo-3-Methoxynicotinate
2-BROMO-3-METHYL-BENZOIC ACID METHYL ESTER
Benzoic acid, 2-bromo-3-methyl-, methyl ester
2-Bromo-m-toluic acid methyl ester (COOCH3=1)
物理化学性质
制备方法
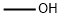
67-56-1

53663-39-1

131001-86-0
将2-溴-3-甲基苯甲酸(5g,23.2mmol)与浓硫酸(0.5mL)在无水甲醇(100mL)中的混合溶液加热回流20小时。反应完成后,通过旋转蒸发仪减压浓缩去除溶剂。将浓缩后的残余物溶解于二氯甲烷(50mL)中,用饱和碳酸氢钠溶液(30mL)洗涤以中和酸性物质。有机相经无水硫酸钠干燥后,过滤并再次通过旋转蒸发仪减压浓缩,得到2-溴-3-甲基苯甲酸甲酯(4.43g,19.3mmol,产率83%)。产物结构经1H NMR(400MHz,CDCl3)确认:δ 2.46(s,3H),3.93(s,3H),7.24(t,J = 8.0Hz,1H),7.34(dd,J = 1.0Hz,J = 8.0Hz,1H),7.46(dd,J = 1.0Hz,J = 8.0Hz,1H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 2, p. 644 - 651
[2] Journal of the American Chemical Society, 2016, vol. 138, # 18, p. 5813 - 5816
[3] Patent: WO2004/37806, 2004, A1. Location in patent: Page 45
[4] Patent: WO2018/205948, 2018, A1. Location in patent: Page/Page column 49
[5] Patent: WO2004/37806, 2004, A1. Location in patent: Page 71
