14510-06-6
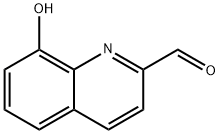 14510-06-6 结构式
14510-06-6 结构式
基本信息
8-羟基-2-喹啉甲醛
8-羟基喹啉-2-甲醛
2-喹啉甲醛,8-羟基-
8-羟基喹啉-2-甲醛 1G
8-羟基喹啉-2-甲醛(98%)
8-hydroxyquinaldaldehyde
Quinaldaldehyde, 8-hydroxy-
8-Hydroxy-2-quinolinecarbaldehyde
8-HYDROXY-QUINOLINE-2-CARBALDEHYDE
8-HYDROXYQUINOLINE-2-CARBOXALDEHYDE
8-Hydroxy-2-quinolinecarboxaldehyde
2-Quinolinecarboxaldehyde, 8-hydroxy-
8-HYDROXYQUINOLINE-2-CARBOXALDEHYDE 98%
8-Hydroxyquinoline-2-carboxaldehyde ,98%
物理化学性质
制备方法
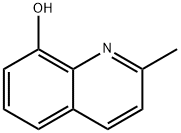
826-81-3

14510-06-6
以8-羟基喹哪啶为原料合成8-羟基喹啉-2-甲醛的一般步骤:制备3,3'-(1E,1'E)-(丙烷-1,3-二基双(氮杂-1-基-1-亚基))双(甲-1-基-1-亚基)二喹啉-8-醇(H2PBIQ)。将8-羟基-2-甲基喹啉(4g,25mmol)、二氧化硒(3.5g,31mmol)、水(3mL)和1,4-二乙二醇(300mL)的混合物在80℃下于氮气保护下搅拌24小时。反应完成后,冷却混合物,过滤并蒸发滤液。通过硅胶柱色谱法(展开溶剂为石油醚:乙酸乙酯,95:5,v/v)纯化所得固体,得到8-羟基喹啉-2-甲醛的纯黄色针状结晶。产率为90%;1H NMR(400MHz,CDCl3):δ10.25(s,1H),8.35(d,J=8.5Hz,1H),8.18(s,1H),8.09(d,J=8.5Hz,1H),7.66(t,J=8.0Hz,1H),7.46(d,J=8.2Hz,1H),7.31(d,J=7.7Hz,1H)。
参考文献:
[1] RSC Advances, 2015, vol. 5, # 129, p. 107012 - 107019
[2] Inorganic Chemistry Communications, 2014, vol. 47, p. 13 - 16
[3] European Journal of Medicinal Chemistry, 2016, vol. 121, p. 864 - 879
[4] Inorganic Chemistry, 2013, vol. 52, # 11, p. 6346 - 6353
[5] Dalton Transactions, 2014, vol. 43, # 26, p. 10164 - 10174
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | H1713 | 8-羟基喹啉-2-甲醛 8-Hydroxyquinoline-2-carbaldehyde | 14510-06-6 | 200mg | 75元 |
| 2025/12/22 | H1713 | 8-羟基喹啉-2-甲醛 8-Hydroxyquinoline-2-carbaldehyde | 14510-06-6 | 1g | 245元 |
| 2025/12/22 | XW145100662 | 8-羟基喹啉-2-甲醛 8-hydroxyquinoline-2-carboxaldehyde;8-hydroxy-2-quinolinecarboxaldehyde | 14510-06-6 | 5G | 2271元 |
