1496-40-8
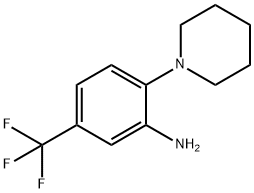 1496-40-8 结构式
1496-40-8 结构式
基本信息
2-PIPERIDIN-1-YL-5-TRIFLUOROMETHYL-PHENYLAMINE
AKOS B025581
AKOS BB-8562
ART-CHEM-BB B025581
ASISCHEM Z43511
BUTTPARK 44\09-69
N-(2-AMINO-4-TRIFLUOROMETHYLPHENYL)PIPERIDINE
2-piperidino-5-(trifluoromethyl)aniline
3-AMINO-4-(1-PIPERIDINO)BENZOTRIFLUORIDE
3-AMINO-4-PIPERIDINOBENZOTRIFLUORIDE
2-(1-Piperidinyl)-5-(trifluoromethyl)aniline
物理化学性质
制备方法
![1-[2-硝基-4-(三氟甲基)苯基]哌啶](/CAS/GIF/1692-79-1.gif)
1692-79-1

1496-40-8
在0℃条件下,向含有1-(2-硝基-4-(三氟甲基)苯基)哌啶(559mg,2.03mmol)的甲醇(10ml)溶液中依次加入浓盐酸(2.00ml,24.0mmol)和无水二氯化锡(2.50g,13.1mmol)。将反应混合物逐渐升温至室温,持续搅拌17.5小时。反应完成后,向混合物中加入饱和碳酸氢钠水溶液进行中和。随后,用乙酸乙酯对混合物进行三次萃取。合并有机层,用盐水洗涤,无水硫酸钠干燥,过滤后减压浓缩。残余物通过硅胶柱色谱法纯化(50g硅胶,洗脱剂为己烷/乙酸乙酯=14/1),得到2-哌啶-1-基-5-(三氟甲基)苯胺(448mg,1.83mmol,收率90.4%)为浅黄色固体。产物经TLC和1H NMR(CDCl3, 400MHz)表征:TLC Rf 0.30(展开剂为己烷/丙酮=18/1);1H NMR(CDCl3, 400MHz)δ 1.59-1.60(m,2H,CH2),1.71(tt,4H,J=5.4,5.4Hz,2CH2),2.85(brs,4H,2CH2),4.09(brs,2H,NH2),6.92(d,1H,J=1.9Hz,芳香-H),6.97(dd,1H,J=1.9,8.4Hz,芳香-H),7.01(d,1H,J=8.4Hz,芳香-H)。
参考文献:
[1] Patent: EP1712242, 2006, A1. Location in patent: Page/Page column 18; 30
[2] Patent: EP1712242, 2006, A1. Location in patent: Page/Page column 18; 31
[3] Patent: EP2279750, 2011, A1
[4] European Journal of Medicinal Chemistry, 2017, vol. 134, p. 97 - 109
[5] Journal of Medicinal Chemistry, 2007, vol. 50, # 4, p. 627 - 640
