151864-81-2
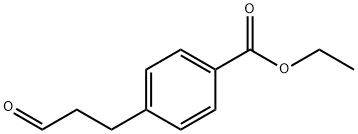 151864-81-2 结构式
151864-81-2 结构式
基本信息
3-(4'-CARBOXYPHENYL)-PROPIONALDEHYDE ETHYL ESTER
4-(3-CARBETHOXY)PHENYLPROPANAL
4-(3-OXO-PROPYL)-BENZOIC ACID ETHYL ESTER
4-CARBOETHOXYPHENYL BUTANAL
4-CARBOETHOXYPHENYL PROPANAL
4-ETHOXYCARBONYLHYDROCINNAMAL
3-(4-Carbethoxy)Phenylpropanal
Benzoic acid, 4-(3-oxopropyl)-, ethyl ester (9CI)
物理化学性质
制备方法

51934-41-9

107-18-6

151864-81-2
以4-碘苯甲酸乙酯(200 g,0.725 mol)和丙烯醇(63 g,1.087 mol)为原料,在搅拌下将其加入到含有碳酸氢钠(152 g,1.812 mol)、四丁基溴化铵(117 g,0.362 mol)和乙酸钯(II)(3.2 g,0.014 mol)的DMF(600 mL)悬浮液中。将反应混合物加热至75-80℃,保持此温度反应3-3.5小时,随后冷却至40-50℃。在剧烈搅拌下,向反应混合物中加入甲苯(1 L),继续搅拌15分钟。将混合物通过硅藻土垫过滤,硅藻土垫用甲苯(2×200 mL)洗涤。合并滤液和洗涤液,用水(3×1 L)洗涤,然后在30-40℃和10 mmHg下蒸发至恒重。得到147.5 g(收率98.8%,HPLC纯度84%)的4-(3-氧代丙基)苯甲酸乙酯粗产物,为深棕色油状物,无需进一步纯化即可用于下一步骤。1H NMR(DMSO-d6):δ 1.38(t,3H),2.81(t,2H),3.03(t,2H),4.39(q,2H),7.27(d,2H),7.98(d,2H),9.81(s,1H)。
参考文献:
[1] Tetrahedron Letters, 1999, vol. 40, # 21, p. 4023 - 4026
[2] Patent: US2005/49296, 2005, A1. Location in patent: Page/Page column 7-8
[3] Organic Letters, 2009, vol. 11, # 22, p. 5342 - 5345
[4] Journal of Heterocyclic Chemistry, 2004, vol. 41, # 6, p. 941 - 946
[5] Patent: WO2006/63863, 2006, A1. Location in patent: Page/Page column 43-44; 1/2
