16948-10-0
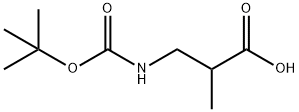 16948-10-0 结构式
16948-10-0 结构式
基本信息
3-[(叔丁氧羰基)氨基]-2-甲基丙酸
3-(BOC-AMINO)-2-METHYLPROPANOIC ACID
REF DUPL: Boc-DL-3-Aminoisobutyric acid
3-(tert-butoxycarbonyl)-2-methylpropanoic acid
N-((tert-Butoxy)carbonyl)-DL-3-aminoisobutyric acid
3-(tert-butoxycarbonylamino)-2-methylpropanoic acid
Boc-DL-3-AMinoisobutyric acid Boc-DL-3-AMinoisobutyric acid
2-methyl-3-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid
Propanoic acid, 3-[[(1,1-diMethylethoxy)carbonyl]aMino]-2-Methyl-, (±)-
物理化学性质
制备方法
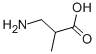
10569-72-9

24424-99-5

16948-10-0
一般步骤:将DL-3-氨基异丁酸(CAS号[10569-72-9],5g,47.52mmol)溶于2N NaOH溶液(24.7mL)中,在冷水浴冷却下,缓慢滴加二碳酸二叔丁酯(Boc2O,11.73g,53.22mmol)的四氢呋喃(THF,75mL)溶液,控制反应温度低于30℃。反应混合物在室温下搅拌18小时后,进行减压浓缩。浓缩物用水(75mL)稀释,并用甲基叔丁基醚(MTBE,3×150mL)洗涤。水相用100g/L柠檬酸溶液(150mL)酸化至pH 3,随后用乙酸乙酯(EtOAc,3×150mL)萃取。合并有机相,用水(3×45mL)洗涤,经无水硫酸镁(MgSO4)干燥,过滤后减压浓缩,得到9.4g目标产物3-((叔丁氧羰基)氨基)-2-甲基丙酸(化合物11),为无色油状物,收率97%。核磁共振氢谱(1H NMR,400 MHz,DMSO-d6,δ,ppm):1.01(d,J = 7.1 Hz,3H);1.38(s,9H);2.48(m,1H);2.91(dt,J = 6.1, 6.5和13.5 Hz,1H);3.15(ddd,J = 6.1, 7.4和13.5 Hz,1H);6.80(t,J = 6.1 Hz,1H);12.15(宽s,1H)。
参考文献:
[1] Journal of Organic Chemistry, 2001, vol. 66, # 20, p. 6541 - 6544
[2] Patent: WO2017/76998, 2017, A1. Location in patent: Page/Page column 163
[3] Patent: EP2380890, 2011, A1. Location in patent: Page/Page column 19
[4] Patent: CN108341752, 2018, A. Location in patent: Paragraph 0482; 0483; 0484; 0485
[5] European Journal of Organic Chemistry, 2012, # 29, p. 5774 - 5788,15
