17159-80-7
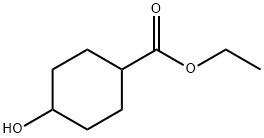 17159-80-7 结构式
17159-80-7 结构式
基本信息
环己基甲酸乙酯
对环己醇甲酸乙酯
4-羟基环己甲酸乙酯
4-羟基环己烷羧酸乙酯
4-(甲酸乙酯)环己醇
4-羟基环己烷甲酸乙酯
4-羟基环己基甲酸乙酯
4-羟基环己烷-1-羧酸乙酯
4-羟基环己烷甲酸乙酯(顺反混合)
Einecs 241-215-1
4-Hydroxycyclohexanecarboxylate
Ethyl 4-hydroxycyclohexanecarbonate
Ethyl 4-hydroxycyclohexylcarboxylate
ETHYL 4-HYDROXYCYCLOHEXANECARBOXYLATE
ethyl 4-hydroxycyclohexane-1-carboxylate
Ethyl4-hydroxycyclohexanecarboxylate,98%
Ethyl 4-hydroxycyclohexanecarboxylate≥ 99% (assay)
4-Hydroxycyclohexane-1-carboxylic acid ethyl ester
物理化学性质
制备方法

17159-79-4
3618-04-0
以4-氧代环己烷羧酸乙酯为原料合成反-4-羟基环己烷甲酸乙酯的一般步骤:在0℃条件下,将4-氧代环己烷羧酸乙酯(13.5g,79mmol)溶于甲醇(150mL)中,缓慢加入硼氢化钠(5.3g,140mmol)。反应混合物在室温下搅拌3小时。反应完成后,加入水(50mL)淬灭反应,随后用乙酸乙酯(150mL×1,50mL×2)进行萃取。合并有机层,用水(50mL)洗涤,无水硫酸钠干燥,过滤后减压浓缩。通过硅胶柱色谱法(洗脱剂:己烷-乙酸乙酯,比例1.5:1至1:1)纯化残余物,得到4-羟基环己烷甲酸乙酯(12g,收率88%,顺反比=3:7),为透明油状物。1H NMR(300MHz,CDCl3,顺式混合物)δ:4.17-4.08(m,2H),3.90(bs,0.3H),3.68-3.57(m,0.7H),2.42-1.28(m,9H),1.27-1.22(m,3H)ppm。(未观察到羟基信号。)
参考文献:
[1] Journal of Medicinal Chemistry, 2016, vol. 59, # 19, p. 8967 - 9004
[2] Organic Process Research and Development, 2010, vol. 14, # 4, p. 883 - 889
[3] Patent: WO2005/80317, 2005, A2. Location in patent: Page/Page column 150
[4] Tetrahedron Letters, 2000, vol. 41, # 39, p. 7567 - 7570
[5] Patent: US5175290, 1992, A
