188345-71-3
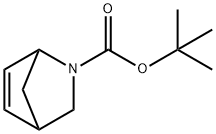 188345-71-3 结构式
188345-71-3 结构式
基本信息
2-氮杂双环<2.2.1>-5-庚烯-2-甲酸
2-BOC-2-氮杂二环[2.2.1]庚-5-烯
N-BOC-2-氮杂双环[2.2.1]庚-5-烯
2-BOC-2-氮杂双环[2.2.1]-5-庚烯
2-氮杂双环<2.2.1>-5-庚烯-2-甲酸叔丁酯
2-氮杂双环[2.2.1]-5-庚烯-2-羧酸叔丁酯
2-BOC-2-氮杂二环[2.2.1]庚-5-烯,98%
2-(叔丁氧羰基)-2-氮杂二环并[2.2.1]庚-5-烯
N-Boc-2-azabicyclo[2.2.1]hept-5-ene
tert-butyl 3-oxo-2-aza-bicyclo[2.2.1]
2-Boc-2-azabicyclo[2.2.1]hept-5-ene,98%
Tert-Butyl2-azabicyclo[2.2.1]hept-5-ene-2-carboxylate
2-(tert-Butoxycarbonyl)-2-azabicyclo[2.2.1]hept-5-ene
tert-butyl 5-azabicyclo[2.2.1]hept-2-ene-5-carboxylate
tert-butyl 2-aza-bicyclo[2.2.1]hept-5-ene-2-carbo×ylate
2-(tert-Butoxycarbonyl)-2-azabicyclo[2.2.1]hept-5-ene
tert-butyl 3-oxo-2-aza-bicyclo[2.2.1]hept-5-ene-2-carboxylate
物理化学性质
制备方法
50-00-0

24424-99-5
542-92-7
![2-氮杂双环[2.2.1]-5-庚烯-2-羧酸叔丁酯](/CAS/GIF/188345-71-3.gif)
188345-71-3
实施例3A 2-氮杂双环[2.2.1]庚-5-烯-2-羧酸叔丁酯的合成:将37%甲醛水溶液(114 mL,1.41 mol)缓慢加入至剧烈搅拌的NH4Cl水溶液(85.0 g,1.59 mol,250 mL水)中。随后,一次性加入新蒸馏的环戊二烯(170 g,2.58 mol),并将反应混合物在室温下持续搅拌17小时。反应完成后,分离下层水相,并用二碳酸二叔丁酯(172 g,0.78 mol)处理。通过滴加1M NaOH水溶液(100 mL)将反应体系的pH调节至约8,并在室温下继续搅拌7小时,期间分批加入固体NaOH(总计40 g)以维持pH在8左右。反应混合物用己烷(2×200 mL)萃取,合并有机相后用盐水(50 mL)洗涤,经无水MgSO4干燥,减压浓缩。残余物通过减压蒸馏纯化,收集沸点为80-92°C/10 mmHg的馏分,得到2-氮杂双环[2.2.1]-5-庚烯-2-甲酸叔丁酯(123 g,0.63 mol,收率45%),为浅黄色液体,冷却后结晶。产物经1H NMR(CDCl3, 300 MHz)和MS(DCI/NH3)表征,数据与预期结构一致。
参考文献:
[1] Journal of Medicinal Chemistry, 2007, vol. 50, # 15, p. 3627 - 3644
[2] Patent: US2005/65178, 2005, A1. Location in patent: Page/Page column 19
[3] Patent: US2015/132258, 2015, A1. Location in patent: Paragraph 0387; 0389; 0390
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | B4806 | 2-(叔丁氧羰基)-2-氮杂二环并[2.2.1]庚-5-烯 2-(tert-Butoxycarbonyl)-2-azabicyclo[2.2.1]hept-5-ene | 188345-71-3 | 1g | 345元 |
| 2025/09/19 | H63698 | 2-Boc-2-氮杂二环[2.2.1]庚-5-烯, 98% 2-Boc-2-azabicyclo[2.2.1]hept-5-ene, 98% | 188345-71-3 | 1g | 1573元 |
| 2025/05/22 | H63698 | 2-Boc-2-氮杂二环[2.2.1]庚-5-烯, 98% 2-Boc-2-azabicyclo[2.2.1]hept-5-ene, 98% | 188345-71-3 | 250mg | 452元 |

