205448-65-3
 205448-65-3 结构式
205448-65-3 结构式
基本信息
7-甲氧基-4-氧代-1
4-二氢喹啉-6-羧酸甲酯
4-氧代-7-甲氧基-1,4-二氢喹啉-6-甲酸甲酯
1,4-二氢-7-甲氧基-4-氧代-6-喹啉羧酸甲酯
7-甲氧基-4-氧代-1,4-二氢喹啉-6-羧酸甲酯
FMX14227-A2
Lenvatinib Intermediate3
Methyl 7-Methoxy-4-oxo-1
4-dihydroquinoline-6-carboxylate
methyl 4-hydroxy-7-methoxyquinoline-6-carboxylate
7-Methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylate
Methyl 7-Methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylate
1,4-Dihydro-7-methoxy-4-oxo-6-quinolinecarboxylic acid methyl ester
7-Methoxy-4-oxo-1,4-dihydro-quinoline-6-carboxylic acid methyl ester
物理化学性质
制备方法
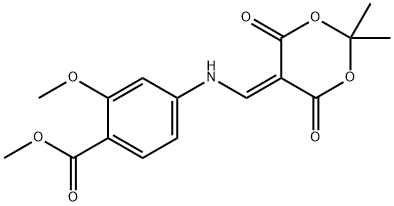
205448-64-2

205448-65-3
以4-((2,2-二甲基-4,6-二氧代-1,3-二噁烷-5-基)甲基氨基)-2-甲氧基苯甲酸甲酯为原料合成7-甲氧基-4-氧代-1,4-二氢喹啉-6-羧酸甲酯的一般步骤:将5-((3-甲氧基-4-甲氧基羰基苯胺基)亚甲基-2,2-二甲基-1,3-二恶烷-4,6-二酮(10g,29.8mmol)悬浮于DOWTHERM A(125ml)中,并在30分钟内加热至180-190℃。原料在100℃下溶解,二氧化碳在约180℃下开始释放。继续加热30分钟后停止加热。随着反应混合物冷却,产物逐渐析出。当温度降至40℃时,加入乙醚并将混合物搅拌30分钟。通过过滤收集固体产物,用乙醚洗涤,并在真空下干燥,得到7-甲氧基-6-甲氧基羰基-1,4-二氢喹啉-4-酮(5.56g,收率80%)。产物的结构通过1H NMR光谱确认:(DMSO-d6)δ 3.80(s,3H);3.85(s,3H);5.95(d,1H);7.00(s,1H);7.85(d,1H);8.40(s,1H);11.6(br s,1H);MS-ESI:234 [MH]+。
参考文献:
[1] Patent: US6809097, 2004, B1. Location in patent: Page column 67
[2] Journal of Medicinal Chemistry, 2008, vol. 51, # 6, p. 1649 - 1667
[3] Journal of Medicinal Chemistry, 2008, vol. 51, # 6, p. 1668 - 1680
[4] Patent: EP1724268, 2006, A1. Location in patent: Page/Page column 48
[5] Patent: EP3293177, 2018, A1. Location in patent: Paragraph 0199; 0201
