220514-28-3
 220514-28-3 结构式
220514-28-3 结构式
基本信息
SWF-37
5-氯-2-甲基苯甲酸甲酯
5-溴-2-甲基-3-硝基苯基乙酸酯
5-溴-2-甲基-3-硝基苯甲酸甲酯
5-溴-3-硝基-2-甲基苯甲酸甲酯
2-甲基-3-硝基-5-溴苯甲酸甲酯
SWF-37
5-broMo-2-Methyl-3-nitrophenyl acetate
Methyl 5-Bromo-2-methyl-3-nitrobenzoate
2-Methyl-5-BroMo-3-nitrobenzoicacidMethylester
5-BROMO-2-METHYL-3-NITROPHENYL METHYLCARBOXYLATE
5-Bromo-2-methyl-3-nitrobenzoic Acid Methyl Ester
Benzoic acid, 5-broMo-2-Methyl-3-nitro-, Methyl ester
5-BROMO-2-METHYL-3-NITROPHENYL METHYLCARBOXYLATE ISO 9001:2015 REACH
物理化学性质
制备方法
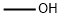
67-56-1

107650-20-4

220514-28-3
以甲醇和2-甲基-3-硝基-5-溴苯甲酸为原料合成5-溴-2-甲基-3-硝基苯甲酸甲酯的一般步骤如下:将2-甲基-3-硝基-5-溴苯甲酸(21.2g,81.5mmol)溶于SOCl2(100mL)中,加热至回流直至混合物变澄清(约1.5小时)。反应完成后,将混合物冷却并真空浓缩。将残余物分批加入250mL甲醇中,所得混合物在回流条件下搅拌过夜。反应完成后,将混合物冷却并浓缩。将残余物溶于300mL乙酸乙酯中,依次用饱和NaHCO3水溶液和盐水洗涤,用MgSO4干燥,浓缩。残余物通过硅胶柱色谱纯化(洗脱剂:石油醚:乙酸乙酯=20:1,v:v),得到5-溴-2-甲基-3-硝基苯甲酸甲酯(21.2g,收率:95%)为浅黄色固体。
参考文献:
[1] Patent: WO2014/18866, 2014, A1. Location in patent: Paragraph 00181; 00183
[2] Patent: US2018/265517, 2018, A1. Location in patent: Paragraph 0318-0321
[3] Patent: US2017/8904, 2017, A1. Location in patent: Paragraph 0422; 0424
[4] Patent: US2018/177750, 2018, A1. Location in patent: Paragraph 1235-1239
[5] Patent: CN105481790, 2016, A. Location in patent: Paragraph 0198; 0199; 0200; 0204
