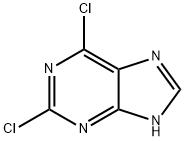
2382-10-7
 2382-10-7 结构式
2382-10-7 结构式
基本信息
2,6-二氯-9-甲基-9H-嘌呤
9-Methyl-2,6-dichloropurine
2,6-DICHLORO-9-METHYL-9H-PURINE
2,6-dichloro-9-methyl-1H-purine
9H-Purine, 2,6-dichloro-9-methyl-
2,6-dichloro-9-methyl-9H-purine176328
物理化学性质
制备方法
5451-40-1
74-88-4

2382-10-7
2,6-二氯-9-甲基-9H-嘌呤(15b)的合成步骤:在0°C下,向2,6-二氯-9H-嘌呤(0.120g,0.635mmol)的无水DMF(1.0mL)溶液中加入碳酸钾(K2CO3,0.270g,1.95mmol),随后加入碘甲烷(0.20mL,3.21mmol)。反应混合物在0℃下持续搅拌5小时。反应完成后,用水淬灭反应,并用乙酸乙酯(EtOAc)萃取。合并有机层,用饱和盐水洗涤,无水硫酸镁(MgSO4)干燥,浓缩后通过硅胶(SiO2)柱色谱法纯化,洗脱梯度从100%己烷至100%乙酸乙酯(EtOAc),得到目标产物15b(83.0mg,0.409mmol,收率64%)为无色固体。产物表征数据如下:IR(ATR,neat)3067, 1554, 1360, 1333, 1223, 1147 cm-1;1H NMR(CDCl3, 600MHz)δ 8.09(s, 1H), 3.89(s, 3H);13C NMR(CDCl3, 150MHz)δ 153.3, 152.7, 151.3, 146.4, 130.4, 30.4。
参考文献:
[1] Patent: WO2014/189830, 2014, A1. Location in patent: Page/Page column 35
[2] ACS Medicinal Chemistry Letters, 2012, vol. 3, # 12, p. 985 - 990
[3] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 12, p. 3486 - 3490
[4] Patent: US2011/237607, 2011, A1. Location in patent: Page/Page column 6; 7
[5] Patent: WO2016/91916, 2016, A1. Location in patent: Page/Page column 66
