25199-84-2
 25199-84-2 结构式
25199-84-2 结构式
基本信息
4-三氟甲基-2-羟基喹啉
4-三氟甲基-2(1H)-喹啉
4-三氟甲基喹啉-2(H)-酮
4-三氟甲基-2(1H)-喹啉酮
4-三氟甲基喹啉-2(1H)-酮
4-(Trifluoromethyl)quinolin-2-ol
4-Trifluoromethyl-1H-quinolin-2-one
4-trifluoroMethyl-2(1H)-quinolinone
4-(trifluoromethyl)quinolin-2(1H)-one
2-HYDROXY-4-(TRIFLUOROMETHYL)QUINOLINE
4--(TrifluoroMethyl)quinoline-2(H)-one
2-hydroxy-4-(trifluoroMethyl)quinqline
2-Hydroxy-4-(trifluoromethyl)quinoline ,98%
物理化学性质
制备方法
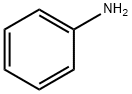
62-53-3

372-31-6

25199-84-2
将苯胺(9.30 g,100 mmol)与4,4,4-三氟-3-氧代丁酸乙酯(36.8 g,200 mmol)混合,于110℃下搅拌反应1小时。反应完成后,对反应混合物进行减压浓缩,随后用蒸馏水(20 mL)稀释。缓慢加入浓硫酸(110 g,1.13 mol),将混合物于90℃下继续搅拌1小时。反应液冷却至室温后,倒入冰水混合物(500 mL)中。通过过滤收集沉淀,并用乙醇(50 mL)进行重结晶,最终得到2-羟基-4-三氟甲基喹啉(9.90 g,收率46.5%)为白色固体。产物经1H NMR(400 MHz,DMSO-d6)表征:δ 12.34(宽峰,1H),7.75(多重峰,2H),7.45(双峰,1H),7.32(多重峰,1H),6.99(单峰,1H)。质谱分析显示m/z 214.20 [M + H]+。
参考文献:
[1] Synthesis, 2003, # 13, p. 2005 - 2010
[2] Patent: WO2018/165385, 2018, A1. Location in patent: Paragraph 00362
[3] European Journal of Organic Chemistry, 2003, # 11, p. 2115 - 2121
[4] Patent: US2012/214803, 2012, A1. Location in patent: Page/Page column 172
[5] Patent: US2008/275057, 2008, A1. Location in patent: Page/Page column 86
