31224-82-5
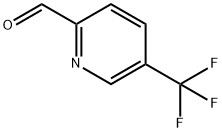 31224-82-5 结构式
31224-82-5 结构式
基本信息
5-(三氟甲基)-2-吡啶甲酸基醛
5-(三氟甲基)吡啶-2-甲醛,95%
Picolinaldehyde, 5-(trifluoromethyl)-
5-Trifluoromethyl-2-pyridinecarbaldehyde
5-Trifluoromethyl-pyridine-2-carbaldehyde
2-Pyridinecarboxaldehyde, 5-(trifluoromethyl)-
5-(Trifluoromethyl)pyridine-2-carboxaldehyde,95%
5-Trifluoromethyl-pyridine-2-carbaldehyde ISO 9001:2015 REACH
5-Trifluoromethyl-Pyridine-2-Carbaldehyde 5-Trifluoromethyl-Pyridine-2-Carbaldehyde
物理化学性质
制备方法

31181-84-7

31224-82-5
以5-三氟甲基吡啶-2-甲醇为原料合成5-三氟甲基吡啶-2-甲醛的一般步骤:将实施例34B(3.89g,22.0mmol)的二氯甲烷(10mL)溶液加入到Dess-Martin periodinane(1,1,1-三(乙酰氧基)-1,1-二氢-1,2-苯并碘氧杂环戊-3(1H)-酮,10.3g,24.2mmol)的二氯甲烷(73mL)溶液中。在氮气保护下,于室温搅拌反应混合物约20分钟,直至固体形成。用50mL二氯甲烷稀释反应混合物,并小心加入约30mL饱和碳酸氢钠溶液以淬灭反应。过滤除去未溶解的固体,将滤液转移至分液漏斗中。水相用二氯甲烷萃取,合并的有机层用盐水洗涤,无水硫酸钠干燥,过滤后减压浓缩至50mL。将浓缩液通过硅胶柱(Analogix? 40×150mm)进行纯化,以25%至50%乙酸乙酯/己烷为洗脱剂。最终得到2.86g(产率74%)的5-三氟甲基吡啶-2-甲醛,为透明油状物,静置后固化。1H NMR(300MHz,CDCl3)δ10.15(s,1H),9.06(m,1H),8.15(m,1H),8.09(d,J=8.1Hz,1H)。
参考文献:
[1] Patent: US2009/124666, 2009, A1. Location in patent: Page/Page column 26
[2] Patent: US2006/223837, 2006, A1. Location in patent: Page/Page column 35
[3] Patent: US2015/232460, 2015, A1. Location in patent: Paragraph 0478-0480
