3160-47-2
 3160-47-2 结构式
3160-47-2 结构式
基本信息
CBZ-L-ASPARTIC ACID 4-METHYL ESTER
N-ALPHA-CARBOBENZOXY-L-ASPARATIC ACID BETA-METHYL ESTER
N-BENZYLOXYCARBONYL-L-ASPARTIC ACID 4-METHYL ESTER
N-CBZ-L-ASPARTIC ACID BETA-METHYL ESTER
(S)-2-BENZYLOXYCARBONYLAMINO-SUCCINIC ACID 4-METHYL ESTER
(S)-2-N-CBZ-AMINO-SUCCINIC ACID 4-METHYL ESTER
Z-ASPARTIC ACID(OME)-OH
Z-ASP(OME)-OH
Z-L-ASPARTIC ACID 4-METHYL ESTER
Z-L-ASPARTIC ACID BETA-METHYL ESTER
N-cbz-L-aspartic acid B-methyl ester
CBZ-L-ASPARTIC ACID BETA METHYL ESTER
Z-L-ASPARTIC ACID B-METHYLESTER
N-carbobenzyloxy-L-aspartic acid beta-methyl ester
N-Cbz-L-aspartic acid β-methyl ester, Z-L-Aspartic acid 4-methyl ester
物理化学性质
制备方法
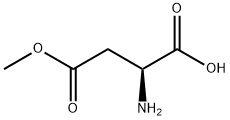
2177-62-0
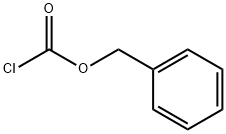
501-53-1

3160-47-2
将30.5 g (207 mmol) (S)-2-氨基-4-甲氧基-4-氧代丁酸溶解于726 mL蒸馏水中,加入260 mL乙醚。随后,加入40.15 g (290 mmol)碳酸钾并搅拌至完全溶解,再缓慢加入41.5 mL (290 mmol)氯甲酸苄酯。反应混合物在室温下持续搅拌4小时。反应完成后,进行相分离,弃去有机层。水层用乙醚洗涤数次,随后用1N盐酸调节pH至1。水层再次用乙醚进行多次萃取,合并有机层并用硫酸镁干燥。最后,通过真空浓缩得到55 g (2S)-4-甲氧基-4-氧代-2-(苯基甲氧基羰基氨基)丁酸,收率为94%。产物经1H NMR (300 MHz, CDCl3)表征:δ 8.23 (s, 1H), 7.35 (m, 5H), 5.87 (d, 1H), 5.12 (s, 2H), 4.69 (m, 1H), 3.68 (s, 3H), 2.97 (dd, 2H)。
参考文献:
[1] European Journal of Organic Chemistry, 2003, # 17, p. 3387 - 3397
[2] Patent: WO2012/148246, 2012, A2. Location in patent: Page/Page column 16
[3] Tetrahedron Letters, 2003, vol. 44, # 28, p. 5251 - 5253
[4] Journal of Organic Chemistry, 2009, vol. 74, # 11, p. 4177 - 4187
[5] Tetrahedron Asymmetry, 2011, vol. 22, # 20-22, p. 1849 - 1854