32499-64-2
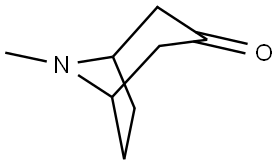
 32499-64-2 结构式
32499-64-2 结构式
基本信息
N-(乙氧羰基调理素酮
3-氧代去甲托烷-8-羧酸乙酯
N-乙氧羰基-4-托品酮
ETHYL 3-OXONORTROPANE-8-CARBOXYLATE
N-CARBETHOXY-4-TROPINONE
N-(CARBOETHOXY)NORTROPINONE
N-(ETHOXYCARBONYL)TROPINONE
3-Oxo-8-azabicylo[3.2.1]octane-8-carboxylic acid
ethyl 3-oxo-8-azabicyclo(3.2.1)octane-8-carboxylate
N-(Carboethoxy)tropinone~3-Oxo-8-azabicyclo[3.2.1]octane-8-carboxylic acid ethyl ester
N-ethoxycarbonylnortropan-3-one
N-(Ethoxycarbonyl)tropinone,99%
n-(carboethoxy)tropinone
3-Oxo-8-azabicylo[3.2.1]octane-8-carboxylic acid ethyl ester
N-(ETHOXYCAZBONYL)NORTROPINONE
N-(Ethoxycarbonyl)nortropinone, 99%
N-CARBETHOXY-4-TROPINONE,98%
物理化学性质
制备方法
532-24-1

541-41-3

32499-64-2
在干燥的500 mL圆底烧瓶中加入搅拌子、8-甲基-8-氮杂二环[3.2.1]辛-3-酮(10.0 g,0.072 mol)和碳酸钾(0.050 g)。加入无水甲苯(90 mL),随后通过注射器缓慢滴加氯甲酸乙酯(21 mL,0.22 mol)。安装冷凝管和氮气鼓泡装置,搅拌下加热至回流,反应过夜。反应完成后,将混合物减压浓缩,得到的棕色油状物溶于二氯甲烷(100 mL)中,用水(100 mL)洗涤。在分液漏斗中分离有机层和水层,水层用二氯甲烷(3×50 mL)萃取。合并有机相,用无水硫酸钠干燥,过滤后减压浓缩。残余物通过硅胶柱色谱纯化(洗脱剂:乙酸乙酯/己烷,1:3),得到3-氧代-8-氮杂双环[3.2.1]辛烷-8-羧酸乙酯(13.1 g,收率92%),为浅黄色油状物。1H NMR (δ): 4.51 (br s, 2H), 4.16 (q, J = 7.2 Hz, 2H), 2.63 (br s, 2H), 2.31 (dd, J = 17.4, 1.6 Hz, 2H), 2.07-2.03 (m, 2H), 1.65 (d, J = 7.6 Hz, 2H), 1.25 (t, J = 7.2 Hz, 3H)。13C NMR (δ): 208.3, 154.1, 61.6, 53.2, 49.1, 29.5 (2), 28.8 (2), 14.9。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2003, vol. 13, # 4, p. 629 - 632
[2] Journal of Heterocyclic Chemistry, 2004, vol. 41, # 4, p. 569 - 574
[3] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 24, p. 7551 - 7558
[4] Patent: CN105294674, 2016, A. Location in patent: Paragraph 0147; 0148; 0149
[5] Patent: WO2018/140876, 2018, A1. Location in patent: Page/Page column 216
