3443-45-6
 3443-45-6 结构式
3443-45-6 结构式
基本信息
1-芘丁酸
1-芘丁酸,97%
1-PYRENEBUTYRIC ACID
4-(1-PYRENYL)BUTYRIC ACID
4-(PYRENE-1-YL)-N-BUTYRIC ACID
1-pyrenebutyrate
pyrene-1-butyric acid
1-PYRENEBUTYRIC ACID, FOR FLUORESCENCE
1-Pyrenebutyricacid,97%
Pyrene-3-butyric acid.
1-Pyrenylbutyric acid
4-(Pyren-1-yl)butyric acid
4-(Pyrene-1-yl)butanoic acid
4-(Pyrene-3-yl)butanoic acid
物理化学性质
安全数据
制备方法
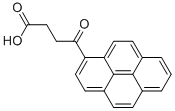
7499-60-7

3443-45-6
以4-氧代-4-(芘-1-基)丁酸为原料合成1-芘丁酸的一般步骤:将1g (2.78mmol) 4-氧代-4-(芘-1-基)丁酸溶解于30mL二甘醇中,加入0.59g (10mmol) 水合肼和0.56g (10mmol) KOH。将反应混合物加热回流2小时,随后倒入冰盐酸(25%)中,生成黄色沉淀。过滤收集固体,并通过乙醇重结晶,得到570mg (71%产率) 4-(芘-1-基)丁酸。分子式C20H16O2,分子量288g/mol。EI-MS: m/z (%) = 289 (16) [MH+], 288 (62) [M], 216 (20), 215 (100), 213 (11)。高分辨EI-MS (C20H16O2): 计算值288.1150; 实测值288.1150。1H-NMR ([D6]-DMSO): δ (ppm) = 8.50-7.92 (9H, m, CHarom), 3.36 (2H, m, CH2C16H9), 2.41 (2H, m, CH2CO2H), 2.04 (2H, m, CH2)。1H-NMR (CDCl3): δ (ppm) = 8.32-7.86 (9H, m, CHarom), 3.42 (2H, t, CH2C16H9, 3JHH = 7.57Hz), 2.51 (2H, t, CH2CO2H, 3JHH = 7.00Hz), 2.23 (2H, m, CH2, 3JHH = 7.57Hz, 3JHH = 7.00Hz)。13C-NMR ([D6]-DMSO): δ (ppm) = 174.74 (1CO), 136.39-123.39 (9 CHarom, 7 Carom), 33.64-26.98 (1CH2CO2H, 2CH2)。13C-NMR (CDCl3): δ (ppm) = 177.48 (1CO), 135.25-123.04 (9 CHarom, 7 Carom), 33.21-26.46 (1CH2CO2H, 2CH2)。IR (KBr): ν (cm-1) = 3447w, 3037w, 2950m, 2934w, 2874w, 1695s, 1431w, 1275m, 1206m, 918w, 846s, 711w。
参考文献:
[1] Patent: US7301043, 2007, B2. Location in patent: Page/Page column 7-8
[2] ACS Catalysis, 2016, vol. 6, # 11, p. 7398 - 7408
[3] New Journal of Chemistry, 2008, vol. 32, # 8, p. 1438 - 1448
[4] Journal of the American Chemical Society, 1941, vol. 63, p. 1682,1684
[5] Justus Liebigs Annalen der Chemie, 1937, vol. 531, p. 1,128
