36692-49-6
 36692-49-6 结构式
36692-49-6 结构式
基本信息
3,4-二氨基苯甲酸甲酯
甲酯3,4-二氨基苯甲酸
BUTTPARK 96\50-24
METHYL 3,4-DIAMINOBENZENECARBOXYLATE
METHYL 3,4-DIAMINOBENZOATE
Methyldibromobenzoate
3,4-DIAMINOBENZOIC ACID METHYL ESTER 97%
3 5-DIAMINO-2-METHYL BENZOIC ACID 97%
BENZOICACID,3,4-DIAMINO-,METHYLESTER
物理化学性质
安全数据
toxic compounds or compounds which causing chronic effects
制备方法
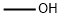
67-56-1
619-05-6

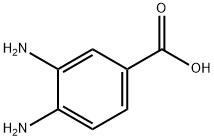
36692-49-6
向3,4-二氨基苯甲酸(1.00 g,6.58 mmol)的甲醇(15.0 mL)溶液中缓慢滴加亚硫酰氯(0.72 mL,9.80 mmol),滴加时间控制在10分钟。反应混合物在室温下搅拌4小时后,减压蒸发除去溶剂。将残余物在水和乙酸乙酯(EtOAc)之间分配,分离有机层。有机层用乙酸乙酯稀释后,依次用饱和碳酸氢钠水溶液、饱和盐水和水洗涤,然后用无水硫酸镁干燥。过滤除去干燥剂,减压蒸发溶剂,得到棕色固体3,4-二氨基苯甲酸甲酯(993 mg,收率95.0%)。产物表征数据如下:1H NMR (CDCl3, 500 MHz) δ 7.49 (d, 1H, J = 7.01 Hz, Ar-H), 7.47 (s, 1H, Ar-H), 6.68 (d, 1H, J = 7.01 Hz, Ar-H), 3.87 (s, 3H, -COOCH3), 3.80 (br s, 2H, -NH2), 3.35 (br s, 2H, -NH2); 13C NMR (CDCl3, 125 MHz) δ 166.56, 140.89, 138.16, 120.87, 118.23, 118.16, 52.11; LCMS m/z 计算值 (MNa)+ 189.06,实测值 189.00。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 24, p. 7456 - 7460
[2] Patent: US2016/33489, 2016, A1. Location in patent: Paragraph 0204
[3] Medicinal Chemistry Research, 2015, vol. 24, # 8, p. 3143 - 3156
[4] Patent: WO2005/113489, 2005, A1. Location in patent: Page/Page column 75-76
[5] Patent: EP1502916, 2005, A1. Location in patent: Page 440
