36745-93-4
 36745-93-4 结构式
36745-93-4 结构式
基本信息
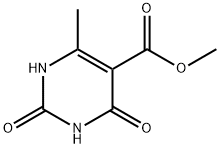
2,4-二氯-6-甲基-5-嘧啶羧酸甲酯
2,4-二氯-6-甲基嘧啶-5-甲酸甲酯
(3-BROMOPHENYL)(3-PYRIDINYL)METHANONE
(S)-methyl 2-(morpholin-3-yl)acetate HCL
Methyl 2,4-dichloro-6-methyl-5-pyrimidinecarboxylate
2,4-Dichloro-6-methyl-5-pyrimidinecarboxylic acid methyl ester
5-Pyrimidinecarboxylic acid, 2,4-dichloro-6-methyl-, methyl ester
2,4-Dichloro-6-methyl-5-pyrimidinecarboxylic acid methyl ester ISO 9001:2015 REACH
methyl 2,4-dichloro-6-methylpyrimidine-5-carboxylatemethyl 2,4-dichloro-6-methylpyrimidine-5-carboxylate
物理化学性质
制备方法
869891-41-8

36745-93-4
以2,4-二羟基-6-甲基吡啶-5-羧酸甲酯为原料合成2,4-二氯-6-甲基嘧啶-5-羧酸甲酯的一般步骤:将6-甲基-2,4-二氧代-1,2,3,4-四氢嘧啶-5-甲酸甲酯(1g,5.43mmol)悬浮于POCl3(10mL)中,加入N,N-二甲基苯胺(10滴)。将反应混合物在105℃下加热6小时,直至形成澄清溶液。反应完成后,将混合物冷却至室温,减压浓缩,随后倒入冰水中,并用乙酸乙酯(EtOAc)萃取。合并有机相,依次用水和饱和盐水洗涤,经无水硫酸钠干燥后,减压浓缩,得到黄绿色固体2,4-二氯-6-甲基嘧啶-5-羧酸甲酯(940mg,收率78%)。质谱分析:[M + H]+ C7H6Cl2N2O2计算值为222,实测值为221、223。
参考文献:
[1] Patent: US2011/152273, 2011, A1. Location in patent: Page/Page column 28
[2] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 24, p. 5947 - 5950
[3] Patent: WO2015/131080, 2015, A1. Location in patent: Paragraph 00270; 00274
[4] Patent: US2014/148454, 2014, A1. Location in patent: Paragraph 0676
[5] Patent: WO2014/82739, 2014, A1. Location in patent: Page/Page column 59
