4046-02-0
 4046-02-0 结构式
4046-02-0 结构式
基本信息
阿魏酸乙酯
BUTTPARK 121\04-55
Ethyl 3-(4-hydroxy-3-methoxyphenyl)acrylate
ETHYL 4-AMINOCINNAMATE
ETHYL 4-HYDROXY-3-METHOXYCINNAMATE
ETHYL FERULATE
ETHYL TRANS-3-(4'-HYDROXY-3'-METHOXYPHENYL)ACRYLATE
FERULIC ACID ETHYL ESTER
RARECHEM AL BI 0735
Ethyl ferulate~Ferulic acid ethyl ester~4-Hydroxy-3-methoxycinnamic acid ethyl ester
(E)-3-(4-HYDROXY-3-METHOXY-PHENYL)-ACRYLIC ACID ETHYL ESTER
Ferulic acid ethyl eser
ETHYL 4-HYDROXY-3-METHOXYCINNAMATE (FERULIC ACID ETHYL ESTER)
3-(3-Methoxy-4-hydroxyphenyl)propenoic acid ethyl ester
3-(4-Hydroxy-3-methoxyphenyl)propenoic acid ethyl ester
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
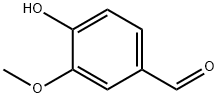
121-33-5
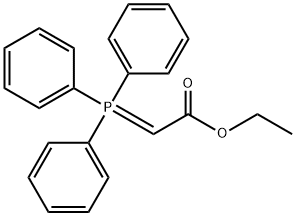
1099-45-2

28028-62-8
以香兰素和乙氧甲酰基亚甲基三苯基膦为原料合成(E)-阿魏酸乙酯的一般步骤:将香兰素(1.5g,9.86mmol)与乙氧甲酰基亚甲基三苯基膦(4.1g,11.8mmol)的苯溶液(35mL)在回流条件下反应4小时。反应完成后(通过TLC监测),在减压下蒸除苯。粗产物通过硅胶柱色谱纯化,使用EtOAc和己烷(4:96)作为洗脱剂,得到(E)-阿魏酸乙酯(2.1g,95%)为无色液体。Rf = 0.50(己烷:EtOAc,9:1);IR(纯)υmax:3430, 2971, 2928, 1710, 1640, 1265, 1170, 1050 cm-1;1H NMR(300MHz,CDCl3):δ 7.62(d,J = 15.8Hz,1H),7.07(dd,J = 1.8, 8.2Hz,1H),7.04-7.03(m,1H),6.92(d,J = 15.8 Hz,1H),6.29(d,J = 15.8 Hz,1H),5.87(s,1H),4.26(q,J = 7.0 Hz,2H),3.39(s,3H),1.33(t,J = 7.2 Hz,3H)ppm;13C NMR(75MHz,CDCl3):δ 167.3, 147.9, 144.6, 126.9, 122.9, 115.5, 114.7, 109.3, 60.3, 55.8, 14.2 ppm;ESIMS m/z实测值425 [M + Na]+(计算值C12H14O4Na,425)。
参考文献:
[1] Organic and Biomolecular Chemistry, 2010, vol. 8, # 22, p. 5199 - 5211
[2] Tetrahedron Letters, 2014, vol. 55, # 32, p. 4427 - 4429
[3] Journal of Medicinal Chemistry, 2009, vol. 52, # 23, p. 7732 - 7752
[4] Patent: US2008/153878, 2008, A1. Location in patent: Page/Page column 17; 24
[5] Molecules, 2011, vol. 16, # 5, p. 3580 - 3596
常见问题列表
1.抗氧化,清除氧自由基,
2.促进血液微循环,
3.防晒
4. 美白祛斑对内皮素ET-1具有拮抗作用,竞争性抑制ET-1与其受体结合,抑制内皮素对黑色素细胞的增殖;
5.抑菌对金黄色葡萄球菌有很好的抑制作用,添加至化妆品可减少防腐剂的使用。
