43038-45-5
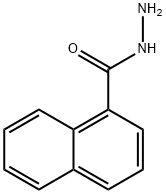 43038-45-5 结构式
43038-45-5 结构式
基本信息
1-萘甲酸肼, 98+%
1-NAPHTHHYDRAZIDE
1-NAPHTHOHYDRAZIDE
1-NAPHTHOIC ACID HYDRAZIDE
1-NAPHTHOIC HYDRAZIDE
AKOS BC-1515
ALPHA-NAPHTHOYLHYDRAZINE
A-NAPHTHOYLHYDRAZINE
SPECS AN-068/40186625
1-Naphthalenecarboxylic acid, hydrazide
1-Naphthoylhydrazine
1-NAPHTHOIC HYDRAZIDE, 98+%
物理化学性质
制备方法

2459-24-7

43038-45-5
以1-萘甲酸甲酯为原料合成1-萘甲酸肼的一般步骤:将1-萘甲酸甲酯(0.52 g,2.8 mmol)溶于15 mL 98%水合肼中,安装空气冷凝管,于110℃搅拌反应8小时。反应完成后,将混合物冷却至室温,过滤收集析出的结晶固体。将所得固体溶于乙酸乙酯(EtOAc)中,依次用水、饱和碳酸氢钠(NaHCO3)溶液和盐水洗涤有机相,然后减压浓缩除去溶剂。通过柱层析法(洗脱梯度:己烷/乙酸乙酯 1:1;己烷/乙酸乙酯 1:2)纯化产物,得到0.38 g(收率73%)白色结晶固体。薄层色谱(TLC)Rf = 0.53(展开剂:氯仿/甲醇 10:1)。熔点166-169℃(文献值166℃)。IR(KBr,cm-1):νmax 3280(N-H),1658(C=O),1606,1588,1524,1261,955。1H NMR(300 MHz,DMSO-d6):δ 9.69(s,1H,NH),8.35-8.10(m,1H,Ar-H),8.05-7.88(m,2H,Ar-H),7.63-7.26(m,4H,Ar-H),4.60(s,2H,NH2)。13C NMR(75 MHz,DMSO-d6):δ 168.2(C=O),133.6,133.3,130.2,130.1,128.4,126.8,126.4,125.6,125.6,125.2(Ar-C)。
参考文献:
[1] European Journal of Medicinal Chemistry, 2016, vol. 120, p. 97 - 110
[2] Journal of the American Chemical Society, 2010, vol. 132, # 20, p. 6900 - 6901
[3] Chemistry - A European Journal, 2011, vol. 17, # 30, p. 8294 - 8298
[4] Bioorganic and Medicinal Chemistry, 2012, vol. 20, # 11, p. 3615 - 3621
[5] RSC Advances, 2015, vol. 5, # 37, p. 28996 - 29001
