465529-56-0
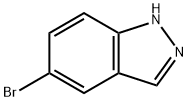
 465529-56-0 结构式
465529-56-0 结构式
基本信息
5-溴-2-甲基-2H-吲唑
2-甲基-5-溴-2H-吲唑
2-Methyl-5-bromoindazole
2-methyl-5-bromo -2H-indazo
5-BROMO-2-METHYL-2H-INDAZOLE
2-Methyl-5-bromo-2H-indazole
2H-INDAZOLE, 5-BROMO-2-METHYL-
物理化学性质
制备方法
53857-57-1
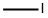
74-88-4

465529-56-0

465529-57-1
向5-溴-1H-吲唑(0.19 g,0.94 mmol)的THF(3 mL)溶液中,在0℃下缓慢加入NaH(0.04 g,1.03 mmol)。反应混合物在该温度下搅拌1小时,随后在0℃下加入碘甲烷(0.09 mL,1.41 mmol)。使反应混合物缓慢升温至室温并继续搅拌2小时。反应完成后,用水淬灭反应,并在减压下浓缩。将残余物用水稀释,并用二氯甲烷(DCM)萃取两次。合并有机层,用无水硫酸钠(Na2SO4)干燥,过滤并浓缩。通过快速柱色谱法(洗脱剂:己烷/乙酸乙酯 = 10:1)纯化粗产物,得到5-溴-1-甲基-1H-吲唑(ii)(88.7 mg,42.5%)和5-溴-2-甲基-2H-吲唑(iii)(60.9 mg,29%),均为白色固体。化合物ii的1H-NMR(400 MHz,DMSO-d6)δ ppm:8.02(d,1H),7.99(d,1H),7.64(d,1H),7.50(dd,1H),4.04(s,3H)。LCMS(方法A):[MH]+ = 211/213,tR = 5.19 min。化合物iii的1H-NMR(400 MHz,DMSO-d6)δ ppm:8.33(s,1H),7.95(d,1H),7.57(d,1H),7.30(dd,1H),4.16(s,3H)。LCMS(方法A):[MH]+ = 211/213,tR = 4.95 min。
参考文献:
[1] Patent: CN105254613, 2016, A. Location in patent: Paragraph 0131; 0132; 0133; 0134; 0135
[2] Journal of Organic Chemistry, 2018, vol. 83, # 12, p. 6334 - 6353
[3] Patent: WO2011/18454, 2011, A1. Location in patent: Page/Page column 60-61
[4] Patent: WO2011/20861, 2011, A1. Location in patent: Page/Page column 59
[5] Chemische Berichte, 1922, vol. 55, p. 1141,1157
