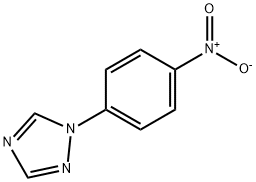
6523-49-5
 6523-49-5 结构式
6523-49-5 结构式
基本信息
1-(4-AMINOPHENYL)-1,2,4-TRIAZOLE
4-[1,2,4]TRIAZOL-1-YLANILINE
4-[1,2,4]TRIAZOL-1-YL-PHENYLAMINE
4-(1H-1,2,4-TRIAZOL-1-YL)ANILINE
AKOS B021934
ART-CHEM-BB B021934
BENZENAMINE, 4-(1H-1,2,4-TRIAZOL-1-YL)-
BUTTPARK 95\50-84
1-(4-Aminophenyl)-1,2,4-triazole 95%
triazolylaniline
物理化学性质
安全数据
制备方法
6219-55-2

6523-49-5
以1-(4-硝基苯基)-1H-1,2,4-三唑(3.0g,15.78mmol)为原料,与氯化铵(2.1g,39.62mmol)共同溶解于50%乙醇-水混合溶剂(60mL)中。随后加入锌粉(2.6g,40mmol),将反应混合物加热回流30分钟。反应完成后,冷却至室温,过滤除去不溶物,滤饼用乙醇(10mL)洗涤。合并滤液,减压浓缩去除溶剂,残余物用水(100mL)稀释,并用乙酸乙酯(100mL×3)进行萃取。有机层用无水硫酸钠干燥,过滤后减压浓缩,得到黄色固体4-(1H-1,2,4-三唑-1-基)苯胺(1.1g,收率:81%),产物无需进一步纯化即可用于后续反应。LC-MS(ESI)分析结果:m/z = 161 [M + H]+。
参考文献:
[1] Chemical and Pharmaceutical Bulletin, 2000, vol. 48, # 12, p. 1935 - 1946
[2] Patent: US2015/336982, 2015, A1. Location in patent: Paragraph 0160; 0162
[3] Applied Spectroscopy, 1995, vol. 49, # 8, p. 1111 - 1119
[4] Patent: US2005/143384, 2005, A1. Location in patent: Page/Page column 23-24
[5] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2012, vol. 51, # 5, p. 731 - 738
