70173-54-5
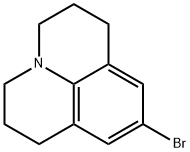 70173-54-5 结构式
70173-54-5 结构式
基本信息
4-溴久洛尼定
9-溴-1,2,3,5,6,7-六氢吡啶并[3,2,1-IJ]喹啉
9-溴-2,3,6,7-四氢-1H,5H-吡啶并[3,2,1-IJ]喹啉
物理化学性质
制备方法

479-59-4
![9-溴-2,3,6,7-四氢-1H,5H-吡啶并[3,2,1-IJ]喹啉](/CAS/GIF/70173-54-5.gif)
70173-54-5
以久洛尼定为原料合成9-溴-1,2,3,5,6,7-六氢吡啶并[3,2,1-ij]喹啉的一般步骤:将久洛尼定(675mg,3.90mmol)和N-溴代琥珀酰亚胺(832mg,4.68mmol)溶于10mL N,N-二甲基甲酰胺(DMF)中,室温搅拌反应5小时。反应完成后,通过硅胶柱色谱法(洗脱剂:己烷/二氯甲烷,体积比3:1)纯化粗产物,得到目标化合物9-溴-1,2,3,5,6,7-六氢吡啶并[3,2,1-ij]喹啉(786mg,收率80%),为油状物。产物结构经以下表征确认:1H NMR(500MHz,CDCl3)δ/ppm:6.87(s,2H),3.10(t,4H,J=5.50Hz),2.70(t,4H,J=6.35Hz),1.94(m,4H);13C NMR(500MHz,CDCl3)δ/ppm:142.0,129.3,123.7,107.3,50.0,27.6,21.9;IR(KBr,cm-1):3045,2935,2836,1581,1506,1462,1446,1310;高分辨质谱(TOFMS-EI)m/z:计算值C12H14NBr [M]+ 251.0310,实测值251.0309。
参考文献:
[1] Chemical and Pharmaceutical Bulletin, 1984, vol. 32, # 5, p. 1770 - 1779
[2] Canadian Journal of Chemistry, 2009, vol. 87, # 2, p. 440 - 447
[3] Journal of Organic Chemistry, 2011, vol. 76, # 22, p. 9391 - 9408
[4] Dyes and Pigments, 2013, vol. 99, # 3, p. 653 - 660
[5] European Journal of Organic Chemistry, 2011, # 30, p. 6100 - 6109
