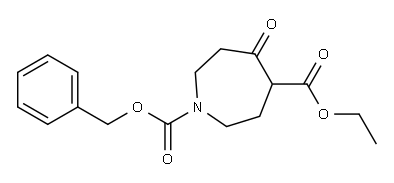
83621-33-4
 83621-33-4 结构式
83621-33-4 结构式
基本信息
N-苄氧羰基-4-氮杂卓酮
N-CBZ-3-氮杂环庚酮
N-CBZ-氮杂环庚烷-4-酮
4-氧代氮杂环庚烷-1-羧酸苄酯
1-CBZ-六氢-4-氧代-1H-氮杂
N-苄氧羰基六氢-1H-氮杂卓-4-酮
N-Cbz-azepan-4-one
N-Cbz Azepane-4-one
4-oxoazepane-1-carboxyL
N-CBZ-4-perhydroazepinone
N-CBZ-PERHYDROAZEPIN-4-ONE
AZEPAN-4-ONE, N-CBZ PROTECTED
N-CBZ-HEXAHYDRO-1H-AZEPIN-4-ONE
1-Cbz-hexahydro-4-oxo-1H-azepine
PERHYDROAZEPIN-4-ONE, N-CBZ PROTECTED
物理化学性质
制备方法
31696-09-0

83621-33-4
以1-Cbz-5-氧代氮杂环庚烷-4-甲酸乙酯为原料合成N-CBZ-4-氮杂卓酮的一般步骤:将氢氧化钾(24.6 g,375 mmol)的水溶液(400 mL)加入到5-苄基4-乙基-5-氧代-1,4-氮杂环二羧酸酯(40.0 g,125 mmol)的乙醇溶液(400 mL)中。将反应混合物加热回流2.5小时。反应完成后,冷却至室温,减压蒸馏除去乙醇。随后,用200 mL盐水和300 mL乙酸乙酯稀释反应混合物。分离有机层和水层,水相用乙酸乙酯(2×100 mL)进一步萃取。合并所有有机层,用盐水洗涤,经无水硫酸镁干燥,减压浓缩得到橙色油状物。通过快速柱色谱法(硅胶,40%乙酸乙酯/己烷为洗脱剂)纯化,得到澄清无色油状产物(22.6 g,收率73%)。产物结构经1H NMR(CDCl3,δ: 7.31-7.30, 5.12, 3.65-3.63, 2.68-2.60, 1.81-1.78)和IR(液体,cm-1: 1698, 1475, 1454, 1442, 1423, 1331, 1320, 1295, 1270, 1241, 1191, 1165, 1091, 900, 699)确认。
参考文献:
[1] Patent: EP1173440, 2004, B1. Location in patent: Page 11
[2] Patent: EP1173440, A1. Location in patent: Page 11
[2] Patent: , 2002, . Location in patent: Page 11
[4] Patent: WO2008/39420, 2008, A2. Location in patent: Page/Page column 32
[5] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 3, p. 918 - 921
