85-79-0
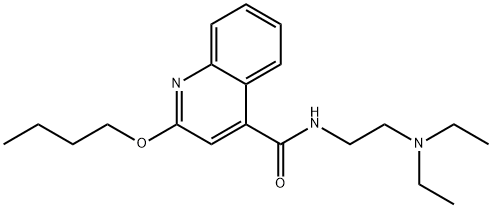 85-79-0 结构式
85-79-0 结构式
基本信息
地布卡因
辛可卡因
辛可(地布)卡因
2-BUTOXY-N-[2-(DIETHYLAMINO)ETHYL]CINCHONAMIDE
2-butoxy-n-(2-diethylaminoethyl)quinoline-4-carboxamide
A-BUTOXYCINCHONINIC ACID DIETHYLETHYLENEDIAMIDE
CINCHOCAINE
DIBUCAINE
DIBUCAINE BASE
2-butoxy-n-(2-(diethylamino)ethyl)-4-quinolinecarboxamid
2-butoxy-n-(2-(diethylamino)ethyl)-cinchoninamid
2-butoxy-n-(2-(diethylamino)ethyl)cinchoninamide
2-butoxy-n-(2-(diethylamino)ethyl-4-quinolinecarboxamid
2-Butoxy-N-(beta-diethylaminoethyl)cinchoninamide
2-Butoxy-N-[2-(diethylamino)ethyl]cinchoninamide
2-Butoxyquinoline-4-carboxylic acid diethylaminoethylamide
2-butoxyquinoline-4-carboxylicaciddiethylaminoethylamide
2-n-butoxy-n-(2-diethylaminoethyl)cinchoninamide
4-Quinolinecarboxamide, 2-butoxy-N-[2-(diethylamino)ethyl]-
alpha-butyloxycinchonicacid-gamma-diethylethylenediamine
alpha-Butyloxycinchoninic acid diethylethylenediamide
alpha-butyloxycinchoninicaciddiethylethylenediamide
物理化学性质
常见问题列表
辛可卡因主要适用于硬膜外麻醉以及腰麻。本品容易通过粘膜,也用于表面麻醉,但因其毒性过大(约比普鲁卡因高15倍),较少用于浸润麻醉。
1、2-氯-N-[2-(二乙基氨基)乙基]-4-喹啉甲酰胺的合成
室温下向3000ml三口瓶中加入2-羟基-4-喹啉羧酸200g,甲苯1500ml搅拌下滴加氯化亚砜158g,升温至75℃反应2小时,降温至25℃,减压浓缩,再加500ml甲苯,继续减压浓缩至干,直接加入2000ml甲苯稀释后加入到5000ml三口瓶中,再加入N,N-二乙基二乙胺100g,升温70℃搅拌,反应完全后降至室温,加水搅拌30分钟,分液,有机层用水洗两次,饱和食盐水洗涤一次,无水硫酸钠干燥,过滤,滤液旋干,得2-氯-N-[2-(二乙基氨基)乙基]-4-喹啉甲酰胺即辛可酰胺274g,收率85%。
2.辛可卡因的合成
将200g辛可酰胺,550ml正丁醇和300g正丁醇钠加入至3000ml反应瓶中,逐渐升温至回流3小时后,降温至室温,加入纯化水1000ml,搅拌30分钟,静止分层30min,将水层弃去,有机层加入无水硫酸钠干燥,过滤,滤液减压浓缩,然后加入500ml甲苯并升温至60℃搅拌30min,静置,分层,上层甲苯层降温析晶,得辛可卡因精制品146g,收率65%,纯度为99.7%。
Dibucaine (Cinchocaine) reduces the degradation of BSA-gold complex in the reservosomes, which was not caused either by an inhibition of the whole proteolytic activity of the parasite or by a reduction on the expression levels of cruzipain. Dibucaine, a quaternary ammonium compound, inhibited SChE to a minimum within 2 min in a reversible manner. The inhibition was very potent. It had an IC(50) of 5.3 microM with BuTch or 3.8 microM with AcTch. The inhibition was competitive with respect to BuTch with a K(i) of 1.3 microM and a linear-mixed type (competitive/noncompetitive) with respect to AcTch with inhibition constants, K(i) and K(I) of 0.66 and 2.5 microM, respectively. Dibucaine possesses a butoxy side chain that is similar to the butryl group of BuTch and longer by an ethylene group from AcTch.

