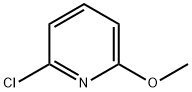
95652-81-6
 95652-81-6 结构式
95652-81-6 结构式
基本信息
2-甲氧基-6-氯吡啶-3-甲醛
6-氯-2-甲氧基-吡啶-3-甲醛
6-Chloro-2-methoxynicotinaldehyde 98%
6-Chloro-2-methoxy-3-pyridinecarbaldehyde
6-CHLORO-2-METHOXY-PYRIDINE-3-CARBALDEHYDE
6-Chloro-2-methoxypyridine-3-carboxaldehyde
6-Chloro-2-methoxy-3-pyridinecarboxaldehyde
3-Pyridinecarboxaldehyde, 6-chloro-2-methoxy-
物理化学性质
安全数据
Eye Irrit. 2
Skin Irrit. 2
Skin Sens. 1
STOT SE 3
制备方法
17228-64-7

68-12-2

95652-81-6
一般步骤:在干燥的氩气氛围下,将2-氯-6-甲氧基吡啶(5g,34.82mmol)溶解于四氢呋喃(THF,100mL)中,冷却至-78℃。缓慢滴加叔丁基锂(tBuLi,1.7M,18.5mL,31.34mmol),保持温度在-78℃,搅拌反应1小时。随后,在相同温度下缓慢加入N,N-二甲基甲酰胺(DMF,92.2g,31.34mmol),继续搅拌2小时。反应完成后,用乙酸淬灭反应,将混合物倒入冰冷的水中。用饱和碳酸氢钠(NaHCO3)溶液碱化水相,随后用乙酸乙酯(EtOAc,2×500mL)萃取。合并有机层,用无水硫酸钠(Na2SO4)干燥,减压浓缩得到粗产物。粗产物通过硅胶柱色谱纯化,洗脱剂为石油醚与乙酸乙酯的混合溶剂(体积比10:1),得到6-氯-2-甲氧基吡啶-3-甲醛,为白色固体(3.8g,收率65%)。质谱(ES+)显示m/z 172 [M+H]+;元素分析(C7H6ClNO2)与理论值相符;1H NMR(300MHz,CDCl3)δ 10.17(s,1H),8.07(d,J=8.04Hz,1H),7.03(d,J=8.04Hz,1H),3.79(s,3H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2017, vol. 60, # 13, p. 5857 - 5867
[2] Patent: US2018/65917, 2018, A1. Location in patent: Paragraph 0209
[3] Patent: EP1405859, 2004, A1. Location in patent: Page 23
[4] Organic Letters, 2014, vol. 16, # 7, p. 1980 - 1983
[5] Chemical and Pharmaceutical Bulletin, 2016, vol. 64, # 7, p. 723 - 732
