11-a-Hydroxy canrenone methyl ester
- CAS No.
- 192704-56-6
- Chemical Name:
- 11-a-Hydroxy canrenone methyl ester
- Synonyms
- 11α-HydroxyMexrenone;11α-Hydroxy Mexrenone;11a-Hydroxy Mexrenone;11α-Hydroxy Mexrenone;Eplerenone intemediate;11alpha-Hydroxymexrenone;11α-Hydroxymexrenone(A2);Eplerenone intermediate A2;11α-Hydroxy canrenone methyl;11α-HydroxEplerenone Impurity
- CBNumber:
- CB11458867
- Molecular Formula:
- C24H32O6
- Molecular Weight:
- 416.51
- MDL Number:
- MFCD18252911
- MOL File:
- 192704-56-6.mol
- MSDS File:
- SDS
| Boiling point | 604.5±55.0 °C(Predicted) |
|---|---|
| Density | 1.27±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 14.40±0.70(Predicted) |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | ZYDNRZOTRVTMRC-ROUXWHDHNA-N |
| SMILES | C1[C@]2(C)[C@@]3([H])[C@H](O)C[C@]4(C)[C@]5(CCC(=O)O5)CC[C@@]4([H])[C@]3([H])[C@H](C(OC)=O)CC2=CC(=O)C1 |&1:1,3,5,8,10,18,20,22,r| |
| CAS DataBase Reference | 192704-56-6 |
11-a-Hydroxy canrenone methyl ester price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | H948140 | 11α-HydroxyMexrenone | 192704-56-6 | 10mg | $150 | 2021-12-16 | Buy |
| Matrix Scientific | 090025 | 11-Alpha-Hydroxy canrenone methyl ester 95+% | 192704-56-6 | 1g | $208 | 2021-12-16 | Buy |
| Matrix Scientific | 090025 | 11-Alpha-Hydroxy canrenone methyl ester 95+% | 192704-56-6 | 5g | $588 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 65434 | 11α-HydroxyMexrenone | 192704-56-6 | 25mg | $650 | 2021-12-16 | Buy |
| AK Scientific | W4125 | 11-a-hydroxycanrenonemethylester | 192704-56-6 | 100mg | $864 | 2021-12-16 | Buy |
11-a-Hydroxy canrenone methyl ester Chemical Properties,Uses,Production
Uses
11a-Hydroxy Mexrenone is an intermediate used in the chemobiological synthesis of Eplerenone (E588775), a cardiovascular drug.
Synthesis

209253-72-5
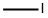
74-88-4

192704-56-6
Under stirring conditions, 5a (5 g, 12 mmol) was dissolved in DMF (40 ml) and heated to 45 °C. Subsequently, AcOH (2.9 ml, 51 mmol) was added to the solution. after 30 min, NaNO2 (2.484 g, 36 mmol) was added slowly in batches. After 1 hour of reaction, DMF (3 ml) solution of AcOH (2.9 ml, 51 mmol) was added dropwise. After 4 hours of reaction, the mixture was cooled to 0°C and quenched with saturated aqueous NaHCO3 solution. Na2SO3 solution (54 ml, 89 mmol) was added. The mixture was warmed to room temperature and stirred overnight. All solvent was removed under vacuum to give a powdered solid. The solid was diluted with acetone, filtered and the filter cake was washed three times with acetone. The organic layer was combined with the filtrate and concentrated to about 100 ml. To the concentrate was added MeI (1.5 ml, 24 mmol) and K2CO3 (5 g, 36 mmol) at 0 °C. The mixture was warmed to room temperature and stirred overnight. The reaction mixture was filtered and the filter cake was washed three times with DCM. The organic layer was combined with the filtrate and all solvents were removed under vacuum. The residue was diluted with DCM, washed sequentially with 10% aqueous HCl, aqueous NaHCO3 and brine, dried with Na2SO4, filtered and the filtrate was concentrated to give the crude product 6. The crude product can be purified by recrystallization from DCM/toluene to give colorless crystals 6 (4 g, 80% yield). The product characterization data were as follows: melting point 230-232 °C; 1H NMR (300 MHz, CDCl3) δ 5.68 (s, 1H), 4.04 (dt, J = 10.23, 9.84, 3.98 Hz, 1H), 3.64 (s, 3H), 2.85-2.71 (m, 2H), 2.68-2.18 (m, 8H), 2.10 -1.57 (m, 9H), 1.49-1.40 (m, 2H), 1.35 (s, 3H), 1.00 (s, 3H); HR-MS (EI) m/z C24H32O6 (M+) calculated value 416.221, measured value 416.225; Elemental analysis of the calculated value (C24H32O6): C 69.21, H 7.74; measured value C 68.32, H 7.72; UV maximum absorption wavelength 244nm.
References
[1] Steroids, 2012, vol. 77, # 11, p. 1086 - 1091


11-a-Hydroxy canrenone methyl ester Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Pharmaceuticals Group Corp., Ltd. | +8613004379774 | wu@jiaerke.com | China | 33 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 | admin01@hsnm.com.cn | China | 2097 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8618531123677 | faithe@yan-xi.com | China | 5853 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9081 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8615713292910 | Nancy@kangcang.com.cn | China | 341 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +852-6619 3215 +86-17731935328 | sales03@chemcn.cn | China | 970 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21620 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29852 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
View Lastest Price from 11-a-Hydroxy canrenone methyl ester manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-09-25 | 11a-Hydroxycanrenone
192704-56-6
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2025-06-20 | 11-a-Hydroxy canrenone methyl ester
192704-56-6
|
US $40.00 / kg | 1kg | 0.99 | 10 tons | Hebei Yanxi Chemical Co., Ltd. | |
 |
2025-03-04 | 11-a-Hydroxy canrenone methyl ester
192704-56-6
|
US $30.00 / box | 1box | 98% | 2000kg | hebei hongtan Biotechnology Co., Ltd |
-

- 11a-Hydroxycanrenone
192704-56-6
- US $1.50 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
-

- 11-a-Hydroxy canrenone methyl ester
192704-56-6
- US $40.00 / kg
- 0.99
- Hebei Yanxi Chemical Co., Ltd.
-

- 11-a-Hydroxy canrenone methyl ester
192704-56-6
- US $30.00 / box
- 98%
- hebei hongtan Biotechnology Co., Ltd
192704-56-6(11-a-Hydroxy canrenone methyl ester)Related Search:
1of4






