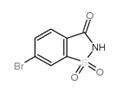
6-BROMOSACCHARINE
- CAS No.
- 62473-92-1
- Chemical Name:
- 6-BROMOSACCHARINE
- Synonyms
- 6-Bromobenzo[d]isothiazol-3(2H)-one 1,1-dioxide;6-bromosaccharin;6-BROMOSACCHARINE;RARECHEM AM UH V180;6-bromo-2H-1???,2-benzothiazole-1,1,3-trione;6-Bromo-1,2-benzisothiazol-3-one 1,1-dioxide;6-bromo-1,2-benzisothiazol-3(2H)-one 1,1-dioxide;1,2-Benzisothiazol-3(2H)-one, 6-bromo-, 1,1-dioxide
- CBNumber:
- CB1443617
- Molecular Formula:
- C7H4BrNO3S
- Molecular Weight:
- 262.08
- MDL Number:
- MFCD03789731
- MOL File:
- 62473-92-1.mol
- MSDS File:
- SDS
| Density | 1.958±0.06 g/cm3(Predicted) |
|---|---|
| storage temp. | Sealed in dry,Room Temperature |
| pka | 1.15±0.30(Predicted) |
| Appearance | White to light yellow Solid |
| InChI | InChI=1S/C7H4BrNO3S/c8-4-1-2-5-6(3-4)13(11,12)9-7(5)10/h1-3H,(H,9,10) |
| InChIKey | CNDPIUXFHSMKMG-UHFFFAOYSA-N |
| SMILES | S1(=O)(=O)C2=C(C=CC(Br)=C2)C(=O)N1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H302-H335-H319 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
6-BROMOSACCHARINE price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ChemScene | CS-W005058 | 6-Bromobenzo[d]isothiazol-3(2H)-one1,1-dioxide | 62473-92-1 | 250mg | $102 | 2021-12-16 | Buy |
| AK Scientific | 8348CR | 6-Bromobenzo[d]isothiazol-3(2H)-one1,1-dioxide | 62473-92-1 | 100mg | $180 | 2021-12-16 | Buy |
| ChemScene | CS-W005058 | 6-Bromobenzo[d]isothiazol-3(2H)-one1,1-dioxide | 62473-92-1 | 1g | $357 | 2021-12-16 | Buy |
| ChemScene | CS-W005058 | 6-Bromobenzo[d]isothiazol-3(2H)-one1,1-dioxide | 62473-92-1 | 100mg | $60 | 2021-12-16 | Buy |
| ChemScene | CS-W005058 | 6-Bromobenzo[d]isothiazol-3(2H)-one1,1-dioxide | 62473-92-1 | 5g | $1001 | 2021-12-16 | Buy |
6-BROMOSACCHARINE Chemical Properties,Uses,Production
Synthesis

135484-83-2

62473-92-1
Methyl 2-amino-4-bromobenzoate (4.5 g, 20 mmol) was stirred in 20% hydrochloric acid (30 mL) until completely dissolved. The solution was cooled to 0 °C, the reaction temperature was controlled not to exceed 5 °C, and a solution of sodium nitrite (1.4 g, 0.020 mol) in water (20 mL) was added dropwise. The mixture was stirred at 0°C for 45 min. Meanwhile, sulfur dioxide was passed into a mixture of acetic acid (50 mL) and water (5 mL) to saturation at 0 °C, followed by the addition of copper (I) chloride (2.0 g, 0.020 mol). The mixture was cooled to 0 °C and the diazonium salt solution was added dropwise over 30 min with vigorous stirring. The reaction mixture was stirred at 0 °C for 1 h. After stirring, the mixture was slowly warmed to room temperature and stirring was continued for 2 h. The mixture was then cooled to room temperature. The mixture was poured into ice water (250 mL) and extracted with EtOAc (3 x 50 mL). The organic phase was washed with saturated NaHCO3 solution and dried over anhydrous Na2SO4. The solvent was concentrated under reduced pressure to give an oily residue, which was dissolved in tetrahydrofuran (40 mL) and cooled to 0 °C. Cold (0°C) 28% ammonium hydroxide solution (40 mL) was added in batches and the reaction temperature was controlled to be below 10°C. The mixture was warmed to room temperature and stirred for 1 hour. The solvent was removed under reduced pressure and the residue was dissolved in saturated aqueous sodium bicarbonate solution (40 mL) and washed with ether (50 mL). The aqueous layer was acidified to pH 1 with concentrated hydrochloric acid, the precipitate was collected by filtration and dried under vacuum to give 6-bromo-1,1-dioxo-1,2-dihydro-1λ6-benzo[d]isothiazol-3-one (500 mg, 10% yield).1H NMR (400 MHz, DMSO) δ 8.44 (d, J=1.5, 1H), 8.04 (dd, J=8.1,1.5, 1H), 7.81 (d, J=8.1,1.5, 1H), 7.81 (d, J=8.1,1.5, 1.1.5, 1.1.5, 1H). 1H), 7.81 (d, J=8.0,1H).
References
[1] Bioorganic and Medicinal Chemistry, 2005, vol. 13, # 4, p. 949 - 961
[2] Patent: US2008/9524, 2008, A1. Location in patent: Page/Page column 410
[3] Patent: US2015/231142, 2015, A1. Location in patent: Paragraph 1267
6-BROMOSACCHARINE Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
6-BROMOSACCHARINE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-2158073036 | info@dakenam.com | China | 13090 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | admin@nexconn.com | China | 10406 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29800 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569262 +86-15003564040 | 1087@dideu.com | China | 3971 | 58 |
| JIAXING REGEN CHEMICAL CO.,LTD | +8613385739570 | regen_chemical1@163.com | China | 226 | 58 |
| Hunan Jibang Biological Technology Co., LTD | +8613776644403 | sales02@kingboomtech.com | China | 1804 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | trendseenbio@gmail.com | China | 11681 | 58 |
| HANGZHOU LEAP CHEM CO., LTD. | +86-571-87711850 | market18@leapchem.com | China | 43339 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131957 | 58 |
| Zhuzhou focus pharmaceutical technology co., LTD | +86-13776644403 | robert@focuspharma.cc | China | 2422 | 58 |
View Lastest Price from 6-BROMOSACCHARINE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2019-12-23 | 6-Bromosaccharin
62473-92-1
|
US $1.00 / KG | 1g | Min98% HPLC | g/kg/ton | Career Henan Chemical Co |
-

- 6-Bromosaccharin
62473-92-1
- US $1.00 / KG
- Min98% HPLC
- Career Henan Chemical Co






