N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
- CAS No.
- 93102-05-7
- Chemical Name:
- N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
- Synonyms
- N-benzyl-1-methoxy-N-((trimethylsilyl)methyl)methanamine;N-(MethoxyMethyl)-1-phenyl-N-(triMethylsilylMethyl)MethanaMine;N-benzyl(methoxy)-N-((trimethylsilyl)methyl)methanamine;)-N-trimethyL;cas:93102-05-7;-N-(methoxymethyL;N-(Methoxymethyl)-N-(trimethyl;N-(Methoxymethyl)-N-(trimethylsilyl) benzylamine;Benzyl-methoxymethyl-trimethylsilanylmethylamine;N-Benzyl-N-methoxymethyltrimethylsilylmethanamine
- CBNumber:
- CB2715045
- Molecular Formula:
- C13H23NOSi
- Molecular Weight:
- 237.41
- MDL Number:
- MFCD00674005
- MOL File:
- 93102-05-7.mol
- MSDS File:
- SDS
| Boiling point | 76 °C0.3 mm Hg(lit.) |
|---|---|
| Density | 0.928 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | 151 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in chloroform, ethyl acetate. |
| form | Liquid |
| pka | 7.29±0.50(Predicted) |
| Specific Gravity | 0.928 |
| color | Clear colorless to light yellow |
| Hydrolytic Sensitivity | 2: reacts with aqueous acid |
| Sensitive | Moisture & Light Sensitive |
| BRN | 4311216 |
| InChIKey | RPZAAFUKDPKTKP-UHFFFAOYSA-N |
| CAS DataBase Reference | 93102-05-7(CAS DataBase Reference) |
| UNSPSC Code | 12352116 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| RIDADR | 1993 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | Ⅲ | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine price More Price(49)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 420697 | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine 96% | 93102-05-7 | 5g | $16.64 | 2024-03-01 | Buy |
| Sigma-Aldrich | 420697 | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine 96% | 93102-05-7 | 25g | $136 | 2024-03-01 | Buy |
| TCI Chemical | B1938 | N-Benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine >98.0%(T) | 93102-05-7 | 5g | $73 | 2025-07-31 | Buy |
| TCI Chemical | B1938 | N-Benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine >98.0%(T) | 93102-05-7 | 25g | $291 | 2025-07-31 | Buy |
| Sigma-Aldrich | 420697 | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine 96% | 93102-05-7 | 100g | $406 | 2024-03-01 | Buy |
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine Chemical Properties,Uses,Production
Preparation
N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine is most conveniently prepared by treatment of benzylamine with chloromethyltrimethylsilane followed by formaldehyde and methanol. Access to higher ether homologs is achieved by replacing methanol with the appropriate alcohol. An alternate procedure involves alkylation of lithium N-benzyltrimethylsilymethylamide with methoxymethyl chloride.
Chemical Properties
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine is clear colorless to light yellow liquid
Physical properties
bp 77–80°C/0.5 mmHg.
Uses
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine is useful reagent in synthesizing N-benzyl substituted pyrrolidines by [3+2] cycloaddition to α,ßunsaturated esters.
Uses
N-Benzyl-N-(methoxymethyl)-
N-trimethylsilylmethylamine (1) is a valuable reagent for in situ
generation of the N-benzyl azomethine ylide (2). It is generally
preferred over alternative silylmethylamine precursors6–8 because
of ease of handling and use. The ylide (2) is most conveniently
generated from (1) using a catalytic amount of trifluoroacetic acid
as described by Achiwa.Alternative catalysts include LiF,
TBAF,Me3SiOTf–CsF, or Me3SiI–CsF. Mechanistic studies
provide evidence that the reactive intermediate generated from
(1) with either CF3CO2H or F? is a 1,3-dipolar species. Reaction
of (2) with alkenes provides an efficient convergent route
to pyrrolidine derivatives. Alkynes afford 3-pyrrolines which can
be converted into pyrroles.The ylide (2) reacts most readily with
electron deficient alkenes and alkynes since this pairing results in
a narrow dipole HOMO–dipolarophile LUMO energy gap.Examples
of suitable dipolarophiles include unsaturated esters,
ketones, imides,nitriles,and sulfones. Cycloaddition occurs
with complete cis stereospecificity (eq 1) which is consistent
with a concerted mechanism. Dipolarophiles containing
an endocyclic double bond afford fused bicyclic pyrrolidines,
whereas substrates with an exocyclic double bond provide access
to spirocyclic systems.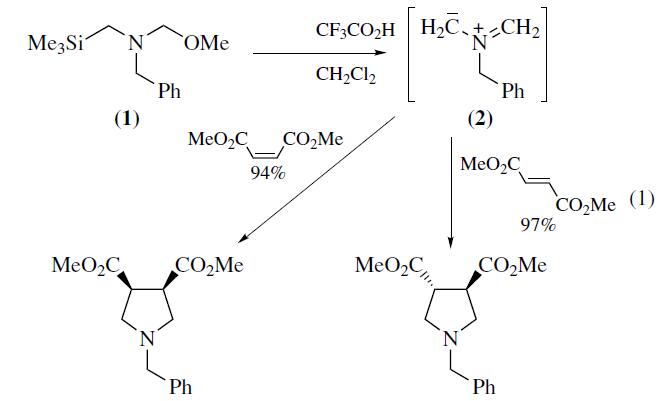
Uses
N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine forms azomethine ylides which readily undergo [3+2] cycloaddition to α,?-unsaturated esters affording N-benzyl substituted pyrrolidines in good yields. It reacts with asymmetric 1,3-dipolar cycloadditions in the practical, large-scale synthesis of chiral pyrrolidines. It is used in the the synthesis of 3-carboxy-1-azabicyclo[2.2.1]heptane derivatives, an important class of physiologically active compounds.
Preparation
most conveniently prepared by treatment of benzylamine with chloromethyltrimethylsilane followed by formaldehyde and methanol.Access to higher ether homologs is achieved by replacing methanol with the appropriate alcohol. An alternate procedure involves alkylation of lithium N-benzyltrimethylsilymethylamide with methoxymethyl chloride.
Synthesis
50-00-0
![N-[(Trimethylsilyl)methyl]benzylamine](/CAS/GIF/53215-95-5.gif)
53215-95-5
93102-05-7
N-[(Trimethylsilyl)methyl]benzylamine (Intermediate P15, 28.588 g, 144.89 mmol) was added dropwise to the formaldehyde solution (37%, 16.816 g, 207.19 mmol) at 0 °C. Subsequently, solid potassium carbonate (16.182 g, 115.91 mmol) was added to the reaction mixture and stirred continuously for 2 hours at room temperature. After completion of the reaction, water (50 mL) was added for dilution and the mixture was extracted with ether (2 x 50 mL). The organic layer was subsequently washed with brine and dried with anhydrous sodium sulfate. Finally, the solvent and desiccant were removed by reduced pressure distillation to afford 33.551 g of the yellow oily target product N-(methoxymethyl)-N-(trimethylsilylmethyl)benzylamine in 97.5% yield. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 7.35-7.18 (m, 5H), 3.99 (s, 2H), 3.75 (s, 2H), 3.22 (s, 3H), 2.18 (s, 2H), 0.13 (s, 9H).
References
[1] Patent: WO2014/24125, 2014, A1. Location in patent: Page/Page column 24-25


N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Zhuangming Biopharma Co., Ltd. | inquiry@zmsilane.com | China | 686 | 58 | |
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 | abby@chuanghaibio.com | China | 8773 | 58 |
| SHANDONG OUCHEM CO.,LTD. | +86-18506360988 | wtaili@ouchem.cn | China | 51 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 880 | 50 |
| Capot Chemical Co.,Ltd. | +8613336195806 | sales@capot.com | China | 29734 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-2158073036 | info@dakenam.com | China | 13091 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21620 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| AB PharmaTech,LLC | 323-480-4688 | sales@acrospharmatech.com | United States | 989 | 55 |
Related articles
- Application of N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
- N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine forms azomethine ylides which readily undergo[3+2] cycloaddition to α,?-un....
- Dec 31,2019
View Lastest Price from N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-07-25 | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
93102-05-7
|
US $0.00-0.00 / KG | 1KG | 95.0% | 10000KGS | Shaanxi Dideu New Materials Co. Ltd | |
 |
2025-06-24 | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
93102-05-7
|
US $0.00-0.00 / KG | 1KG | 99% | 20 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-04-04 | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
93102-05-7
|
US $0.00 / KG | 25KG | 97% | 1tone | Henan Aochuang Chemical Co.,Ltd. |
-

- N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
93102-05-7
- US $0.00-0.00 / KG
- 95.0%
- Shaanxi Dideu New Materials Co. Ltd
-

- N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
93102-05-7
- US $0.00-0.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine
93102-05-7
- US $0.00 / KG
- 97%
- Henan Aochuang Chemical Co.,Ltd.






