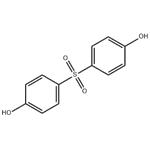
Bis(4-hydroxyphenyl) Sulfone
- CAS No.
- 80-09-1
- Chemical Name:
- Bis(4-hydroxyphenyl) Sulfone
- Synonyms
- BISPHENOL S;SDP;4,4-DIHYDROXYDIPHENYL SULPHONE;4,4-SULFONYLDIPHENOL;-Hexabromodiphenyl Ether-13C6;50kg;bps1;3-dione;diphonec;-TetraCDE
- CBNumber:
- CB4306294
- Molecular Formula:
- C12H10O4S
- Molecular Weight:
- 250.27
- MDL Number:
- MFCD00002350
- MOL File:
- 80-09-1.mol
- MSDS File:
- SDS
| Product description | Number | Pack Size | Price |
| 4,4′-Sulfonyldiphenol 98% | 103039 | 100g | $48.7 |
| 4,4′-Sulfonyldiphenol 98% | 103039 | 500g | $171 |
| Bis(4-hydroxyphenyl) sulfone for synthesis | 8.03258 | 100g | $43.2 |
| Bis(4-hydroxyphenyl) sulfone for synthesis | 8.03258 | 500g | $85.4 |
| Bis(4-hydroxyphenyl) Sulfone >98.0%(HPLC)(T) | B0495 | 25g | $21 |
| More product size | |||
| Melting point | 245-250 °C (lit.) |
|---|---|
| Boiling point | 363.4°C (rough estimate) |
| bulk density | 500kg/m3 |
| Density | 1.366 |
| vapor pressure | <0.0001 Pa (20 °C) |
| refractive index | 1.5220 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | 1.1g/l |
| pka | 7?+-.0.15(Predicted) |
| form | Crystalline Powder |
| color | White to grayish-green |
| Odor | odorless |
| PH | 6.6-7.0 (100g/l, H2O, 20℃) |
| Water Solubility | 1.1 g/L (20 ºC) |
| λmax | 295nm(H2O)(lit.) |
| BRN | 2052954 |
| Stability | Stable. Incompatible with strong bases, acid chloride, acid anhydrides, strong oxidizing agents. |
| Major Application |
cleaning products cosmetics food and beverages personal care |
| InChI | 1S/C12H10O4S/c13-9-1-5-11(6-2-9)17(15,16)12-7-3-10(14)4-8-12/h1-8,13-14H |
| InChIKey | VPWNQTHUCYMVMZ-UHFFFAOYSA-N |
| SMILES | Oc1ccc(cc1)S(=O)(=O)c2ccc(O)cc2 |
| LogP | 1.2 at 23℃ |
| Indirect Additives used in Food Contact Substances | DIPHENYLSULFONE |
| CAS DataBase Reference | 80-09-1(CAS DataBase Reference) |
| FDA UNII | 3OX4RR782R |
| EPA Substance Registry System | Phenol, 4,4'-sulfonylbis- (80-09-1) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H402-H303-H315-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a-P264-P273-P280-P305+P351+P338+P337+P313-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36-36/37/38 | |||||||||
| Safety Statements | 26-39-36-37 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SM8925000 | |||||||||
| Autoignition Temperature | >=400 °C | |||||||||
| TSCA | TSCA listed | |||||||||
| HS Code | 29309070 | |||||||||
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
|||||||||
| Hazard Classifications | Repr. 1B | |||||||||
| Hazardous Substances Data | 80-09-1(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 2830 mg/kg | |||||||||
| NFPA 704 |
|
Bis(4-hydroxyphenyl) Sulfone price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 103039 | 4,4′-Sulfonyldiphenol 98% | 80-09-1 | 100g | $48.7 | 2025-07-31 | Buy |
| Sigma-Aldrich | 103039 | 4,4′-Sulfonyldiphenol 98% | 80-09-1 | 500g | $171 | 2025-07-31 | Buy |
| Sigma-Aldrich | 8.03258 | Bis(4-hydroxyphenyl) sulfone for synthesis | 80-09-1 | 100g | $43.2 | 2023-06-20 | Buy |
| Sigma-Aldrich | 8.03258 | Bis(4-hydroxyphenyl) sulfone for synthesis | 80-09-1 | 500g | $85.4 | 2023-06-20 | Buy |
| TCI Chemical | B0495 | Bis(4-hydroxyphenyl) Sulfone >98.0%(HPLC)(T) | 80-09-1 | 25g | $21 | 2025-07-31 | Buy |
Bis(4-hydroxyphenyl) Sulfone Chemical Properties,Uses,Production
Description
Bisphenol S, also known as 4,4'-Sulfonyldiphenol, abbreviated as BPS, is a synthetic bisphenol (BP) compound. It was originally used as a safe alternative to bisphenol A and is present in many household products, including food packaging, beverage containers, lotions, toys, plastic PVC floors and water pipes[1]. However, current studies have found that BPS does not seem to be very safe and may damage the function of melanocytes[2]. Higher BPS exposure is also associated with increased serum uric acid concentrations, and this association is more obvious in boys, which poses certain health hazards[3].
Chemical Properties
White needle-like crystals. Freely soluble in aliphatic hydrocarbons, soluble in alcohols and ethers, slightly soluble in aromatics, insoluble in water. Bisphenol S molecule contains two hydroxyl groups and a strong electron-absorbing sulfone group, so the acidity is stronger than other phenols.
Uses
4,4'-Sulfonyldiphenol is commonly used as a reactant in epoxy reactions and is also used as a latent thermal catalyst for epoxy resin.
Uses
Bis(4-hydroxyphenyl) Sulfone is commonly used as a reactant in epoxy reactions and is also used as a latent thermal catalyst for epoxy resin.
Preparation
A process for the preparation of bis(4-hydroxyphenyl)sulfone by reacting phenol with sulfuric acid in the presence of a solvent at from 130° to 220° C and in the presence or absence of a sulfonating assistant.
Definition
ChEBI: 4,4'-sulfonyldiphenol is a sulfone that is diphenyl sulfone in which both of the para hydrogens have been replaced by hydroxy groups. It has a role as a metabolite and an endocrine disruptor. It is a sulfone and a bisphenol. It is functionally related to a diphenyl sulfone.
General Description
Bisphenol S (BPS), belonging to the class of bisphenols, is a structural analog of bisphenol A (BPA), used in a variety of industrial applications and products marketed as BPA-free. It is commonly identified in food and beverages, environmental samples, biological matrices, etc. It is also reported to be used in the production of epoxy resins. BPS is also found to be stable compared to BPA at elevated temperature and sunlight conditions.
Health Hazard
Although there is no direct link established between bis(4-hydroxyphenyl) sulfone(BPS) and cardiac disease, it is thought that bis(4-hydroxyphenyl) sulfone may operate by a similar mechanism to BPA and could cause cardiac toxicity.
Flammability and Explosibility
Non flammable
Synthesis

108-95-2

80-09-1

5397-34-2
Under stirring conditions, 100 g (1.0 mol) of sulfuric acid at a concentration of 98% was slowly added dropwise to a mixed system containing 144 g of homotrimethylbenzene, 189 g (2.0 mol) of phenol and 11.9 g (0.05 mol) of benzene-1,3-disulfonic acid. The reaction mixture was heated in an oil bath at 200°C. As the temperature approached 145°C, the reaction mixture began to boil. The distillate was condensed by means of a condenser and separated into two phases by means of a trap. The upper organic phase is continuously returned to the reaction system. The distillation process continued for 5 hours before the temperature of the reaction mixture reached 165°C, at which time the volume of the lower aqueous phase separated by the trap stabilized at 38 mL. Crystal precipitation was observed in the reaction system, forming a slurry. A small amount of the reaction slurry was analyzed by high performance liquid chromatography (HPLC), which showed a weight ratio of 4,4'-bisphenol S (4,4'-BS), 2,4'-dihydroxydiphenylsulfone (2,4'-BS), and trihydroxytriphenyldisulfone of 96.0:3.0:1.0. Subsequently, post-processing was carried out in accordance with the method of Example 1 , resulting in 237 g of crystal product. The composition (weight ratio) of this crystal product was 4,4'-BS:2,4'-BS:trihydroxytriphenyl disulfone = 99.5:0.5:0. The yield of 4,4'-BS was 94% based on starting sulfuric acid. No sulfonic acids were detected in the crystal product, which consisted mainly of benzene-1,3-disulfonic acid and phenol sulfonic acid.
References
[1] J. APAU Eric A Akwasi Acheampong. Exposure to bisphenol A, bisphenol F, and bisphenol S can result in obesity in human body[J]. Cogent Chemistry, 2018. DOI:10.1080/23312009.2018.1506601.
[2] GOENKA S. Disruption of functions of primary human neonatal melanocytes cultured in the presence of bisphenol A and its analogs bisphenol F and bisphenol S[J]. Journal of hazardous materials letters, 2024, 5: Article 100110. DOI:10.1016/j.hazl.2024.100110.
[3] Y. LEE. Relationship between bisphenol A, bisphenol S, and bisphenol F and serum uric acid concentrations among school-aged children[J]. PLoS ONE, 2022. DOI:10.1371/journal.pone.0268503.
Bis(4-hydroxyphenyl) Sulfone Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Changzhou waston chemical technology Co.,Ltd | +86-051985861892 +8618112881323 | info@wastonchem.com | China | 314 | 58 |
| Weifang Dayoo Biochemical Co., Ltd., | +undefined13305362020 | sales@dayoochemical.com | China | 20 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615350571055 | Sibel@chuanghaibio.com | China | 8753 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8618531123677 | faithe@yan-xi.com | China | 5853 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5242 | 58 |
| Shanghai Qyubiotech Co., Ltd. | +86-086-021-37596619 +8618019359838 | info@qyubiotech.com | China | 307 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18126 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20128 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
Related articles
- Exploring the Properties, Applications, and Safety of Bis(4-hydroxyphenyl) Sulfone
- Bis(4-hydroxyphenyl) sulfone is a white crystalline compound used in various industries. It is used as an intermediate and ele....
- Jan 4,2024
- Bis(4-hydroxyphenyl) Sulfone: unveiling the toxic effects and reproductive disruptions of a common compound
- Recent scientific research has shed light on the toxic effects and reproductive disruptions caused by a commonly used compound....
- Jul 12,2023
- Uses of Bisphenol S
- 4,4'-dihydroxydiphenyl sulfone is mainly used as a fixing agent. In addition, it can be used as a plating solution additive, a....
- Oct 23,2019
View Lastest Price from Bis(4-hydroxyphenyl) Sulfone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2026-01-24 | 4,4'-Sulfonyldiphenol
80-09-1
|
0.99 | RongNa Biotechnology Co.,Ltd | ||||
 |
2026-01-23 | 4,4'-Sulfonyldiphenol
80-09-1
|
US $0.00 / Kg/Bag | 1KG | 99% | 5000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2026-01-23 | Bis(4-hydroxyphenyl) Sulfone
80-09-1
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- 4,4'-Sulfonyldiphenol
80-09-1
- 0.99
- RongNa Biotechnology Co.,Ltd
-

- 4,4'-Sulfonyldiphenol
80-09-1
- US $0.00 / Kg/Bag
- 99%
- Jinan Finer Chemical Co., Ltd
-

- Bis(4-hydroxyphenyl) Sulfone
80-09-1
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD





