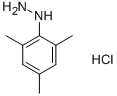
1-Mesitylhydrazine hydrochloride
- CAS No.
- 24006-09-5
- Chemical Name:
- 1-Mesitylhydrazine hydrochloride
- Synonyms
- CBNumber:
- CB52491742
- Molecular Formula:
- C9H14N2.ClH
- Molecular Weight:
- 186.684
- MDL Number:
- MFCD00052268
- MOL File:
- 24006-09-5.mol
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H315-H335-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P-P264-P270-P301+P310-P321-P330-P405-P501 | |||||||||
| NFPA 704 |
|
1-Mesitylhydrazine hydrochloride price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | T808513 | 2,4,6-Trimethylphenylhydrazine hydrochloride | 24006-09-5 | 2.5mg | $45 | 2021-12-16 | Buy |
| TRC | T808513 | 2,4,6-Trimethylphenylhydrazine hydrochloride | 24006-09-5 | 5mg | $60 | 2021-12-16 | Buy |
| TRC | T808513 | 2,4,6-Trimethylphenylhydrazine hydrochloride | 24006-09-5 | 25mg | $75 | 2021-12-16 | Buy |
| Ark Pharm | AK116106 | Mesitylhydrazinehydrochloride 95% | 24006-09-5 | 1g | $22 | 2021-12-16 | Buy |
| Ambeed | A153688 | Mesitylhydrazinehydrochloride 95% | 24006-09-5 | 250mg | $8 | 2021-12-16 | Buy |
1-Mesitylhydrazine hydrochloride Chemical Properties,Uses,Production
Synthesis

88-05-1
24006-09-5
The general procedure for the synthesis of 2,4,6-trimethylphenylhydrazine hydrochloride from homotrimethylaniline was as follows: concentrated hydrochloric acid (0.125 L) and deionized water (0.100 L) were added to a 3.0 L three-necked, round-bottomed flask fitted with a mechanical stirrer and dosing funnel. The solution was cooled to -5 °C in an ice/brine bath and 2,4,6-trimethylaniline (70 mL, 0.50 mol, 1.0 eq.) was added slowly and dropwise over a 30-minute period; formation of a white precipitate was observed during this time. Stirring of the mixture was continued for 15-20 minutes, followed by the dropwise addition of 52.5 mL of a 9.5 M aqueous sodium nitrite solution (70 mL, 0.50 mol, 1.0 equiv) over 30 to 45 minutes. The reaction mixture was stirred for 30 minutes to form a brown solution. A solution of 3.3 M stannous chloride dihydrate in 1:1 concentrated hydrochloric acid and water (0.455 L, 1.5 mol, 2.5 eq.) was added slowly over a period of 4 hr. with vigorous stirring. (Note: Upon addition of the stannous chloride solution, a thick, light orange slurry will form which may cause the mechanical stirrer to clog. A small amount of deionized water may be added at this time to restore stirring.) Gradually warm the mixture to room temperature and continue stirring vigorously for 16 hours. Subsequently, the mixture was cooled to 0 °C and held for 1 h. The mixture was filtered through a fine pore glass sintered Büchner funnel and the collected orange solid was washed with brine and ether. The orange solid was transferred to a 2L round-bottomed flask containing 10M NaOH aqueous solution (1.0 L) and ether (0.700 L) and cooled to -5°C. After the solid was almost completely dissolved (about 1 hour), the organic layer was separated and the aqueous layer was extracted with ether (2 x 0.400 L). The organic phases were combined, dried over anhydrous Na2SO4, filtered into a flame-dried 2.0 L round-bottomed flask, placed under a nitrogen atmosphere, cooled to 0°C, and treated with a 1,4-dioxane solution (0.125 L) in 4 M HCl. The white precipitate formed was collected by vacuum filtration, washed with ether and recrystallized from 200 standard ethanol to give the final 2,4,6-trimethylphenylhydrazine hydrochloride (50 g, 0.27 mol, 54% yield) as a white powder. The product was analyzed by 1H NMR (400 MHz, DMSO) δ 9.57 (3H, s, br, NH, exchangeable), 6.89 (s, 2H), 2.33 (s, 6H), 2.21 (s, 3H); 13C NMR (100 MHz, DMSO) δ 138.06,136.20,135.01,129.17,20.54, 17.95; IR (thin film) v 3294,3002,2964,2911,2680,1564,1513,1481,852,825,753 cm-1; HRESI+/TOF-MS calculated value of C9H15N2+ [M]+ 151.1230, and the measured value of 134.0921 corresponds to the aniline anion radical ( 134.0975).
References
[1] Patent: WO2007/124494, 2007, A2. Location in patent: Page/Page column 54-55
[2] Tetrahedron, 2016, vol. 72, # 17, p. 2186 - 2195
[3] Organic Syntheses, 2010, vol. 87, p. 362 - 376
1-Mesitylhydrazine hydrochloride Preparation Products And Raw materials
1-Mesitylhydrazine hydrochloride Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23541 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +86-18621343501; +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | tp@aladdinsci.com | United States | 52924 | 58 | |
| Shandong Liteng Biotechnology Co. , Ltd. | +86-18663721413 +86-15552573001 | rosy@litengpharm.com | China | 3285 | 58 |
| Compound Net Biotechnology Inc. | +8615303909093 | sales@compound-net.com | China | 2914 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131957 | 58 |
| Shanghai Hanhong Scientific Co.,Ltd. | 021-54306202 13764082696 | info@hanhongsci.com | China | 42943 | 64 |
| Thermo Fisher Scientific | 800-810-5118 | cnchemical@thermofisher.com | China | 17770 | 75 |
| Bide Pharmatech Ltd. | 400-164-7117 13681763483 | product02@bidepharm.com | China | 42446 | 60 |






