1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
- CAS No.
- 141556-45-8
- Chemical Name:
- 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
- Synonyms
- IMes.HCl;IMESCL;1,3-DiMesityl-1H-iMidazol-3-iuM chloride;1,3-Bis(mesityl)imidazolium chloride;1,3-BIS(2,4,6-TRIMETHYLPHENYL)-4,5-DIHYDROIMIDAZOLIUM CHLORIDE;1,3-Bisimidazolium chloride;1,3-DIMESITYLIMIDAZOLIUM CHLORIDE;1,3-DimesitylimidazoliumChloride>1,3-Dimesitylimidazoliumchloride,96%;1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLI&
- CBNumber:
- CB5707153
- Molecular Formula:
- C21H25ClN2
- Molecular Weight:
- 340.89
- MDL Number:
- MFCD02684541
- MOL File:
- 141556-45-8.mol
- MSDS File:
- SDS
| Melting point | >300 °C (lit.) |
|---|---|
| Boiling point | 499.2°C (rough estimate) |
| Density | 1.0279 (rough estimate) |
| refractive index | 1.5940 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| form | Powder |
| color | Off-white to beige |
| Water Solubility | Slightly soluble in water. |
| InChI | InChI=1S/C21H25N2.ClH/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;/h7-13H,1-6H3;1H/q+1;/p-1 |
| InChIKey | OTOSIXGMLYKKOW-UHFFFAOYSA-M |
| SMILES | N1(C=C[N+](C2C(C)=CC(C)=CC=2C)=C1)C1C(C)=CC(C)=CC=1C.[Cl-] |
| CAS DataBase Reference | 141556-45-8(CAS DataBase Reference) |
| UNSPSC Code | 12352005 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | No | |||||||||
| HS Code | 29332900 | |||||||||
| NFPA 704 |
|
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 574066 | 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride | 141556-45-8 | 1g | $89.4 | 2025-07-31 | Buy |
| Sigma-Aldrich | 574066 | 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride | 141556-45-8 | 5g | $272 | 2025-07-31 | Buy |
| TCI Chemical | D3446 | 1,3-Dimesitylimidazolium Chloride >98.0%(HPLC) | 141556-45-8 | 1g | $68 | 2025-07-31 | Buy |
| TCI Chemical | D3446 | 1,3-Dimesitylimidazolium Chloride >98.0%(HPLC) | 141556-45-8 | 5g | $217 | 2025-07-31 | Buy |
| Strem Chemicals | 07-0299 | 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride, min. 97% | 141556-45-8 | 1g | $67 | 2024-03-01 | Buy |
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride Chemical Properties,Uses,Production
Chemical Properties
off-white to beige powder
Uses
1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride is used as a phosphine-free ligand in various metal-catalyzed coupling reactions, often with advantageous results in difficult cases. For use in the Pd-catalyzed cross-coupling of aryl Grignards with aryl chlorides (Kumada reaction). Many examples have been recorded of the use of NHC ligands in the Suzuki coupling reaction, for examples utilizing 1,3-dimesitylimidazol-2-ylidene, in the coupling of arylboronic acids with relatively unreactive aryl chlorides.
Preparation
In a flask, the imine(3 g, 10 mmol) was dissolved in tetrahydrofuran(25 ml), followed by dropwise addition of chloromethyl ethyl ether(1.04 g, 11 mmol). the mixture was stirred under N2 at 40 °C for 18 h, and then ethyl ether(25 ml) was added to separate white solid. The solid was filtered and washed with ethyl ether. Finally, the white solid was dried under vacuum affording 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride.
Reactions
Precursor to the nucleophilic carbene that serves as a bulky, electron-rich "phosphine mimic" for metal-catalyzed reactions.
(a) Palladium-catalyzed Suzuki cross-coupling of aryl chlorides.
(b) Palladium-catalyzed Kumada cross-coupling of aryl chlorides.
(c) Ruthenium-carbene catalysts for ring-closing metathesis.
(d) Suzuki coupling of aryltrimethylammonium salts.
(e) Sonogashira coupling of aryl bromides.
Precursor to a nucleophilic carbene that serves as catalyst.
Ligand for arylation of aldehydes.
Ligand for carbene catalyzed intermolecular arylation of C-H bonds.
Catalyst for boron conjugate additions to cyclic and acyclic α,β-unsaturated carbonyls.
Ligand for dehydrogenative cyclocondensation of aldehydes, alkynes, and dialkylsilanes.
Precursor for carbene for conjugate silylation of alpha, beta-unsaturated carbonyls.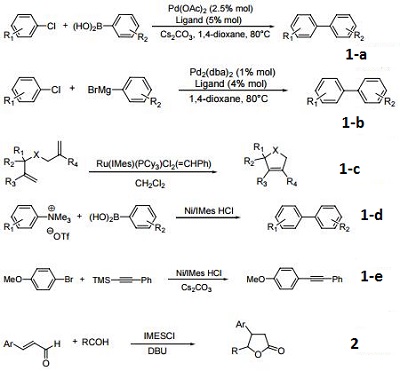


reaction suitability
reagent type: ligand
Synthesis

56222-36-7

3188-13-4

141556-45-8
The general procedure for the synthesis of 1,3-bis(2,4,6-trimethylphenyl)imidazolium chloride from N,N'-bis(2,4,6-trimethylphenyl)ethane-1,2-diimine and chloromethyl ethyl ether is as follows: in a dry flask, N,N'-bis(2,4,6-trimethylphenyl)ethane-1,2-diimine (3 g, 10 mmol) was dissolved in anhydrous tetrahydrofuran ( 25 ml) followed by slow dropwise addition of chloromethyl ethyl ether (1.04 g, 11 mmol). The reaction mixture was stirred at 40 °C for 18 h under nitrogen protection. After completion of the reaction, ether (25 ml) was added to precipitate a white solid. The solid product was collected by filtration and washed with ether and finally dried under vacuum to afford 1,3-bis(2,4,6-trimethylphenyl)imidazole chloride (IMes-HCl) 2.2 g in 65% yield. The structure of the product was confirmed by 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz): 1H NMR δ 2.15 (s, 12H), 2.33 (s, 6H), 7.00 (s, 4H), 7.70 (s, 2H), 10.73 (s, 1H); 13C NMR δ 17.6, 21.1, 124.8, 129.8, 130.7, 134.1, 139.2, 141.1.
References
[1] Journal of Organic Chemistry, 2008, vol. 73, # 7, p. 2784 - 2791
[2] Tetrahedron Letters, 2012, vol. 53, # 7, p. 815 - 818
[3] Tetrahedron, 2012, vol. 68, # 38, p. 7949 - 7955
[4] Chemical Communications, 2011, vol. 47, # 5, p. 1559 - 1561
[5] Journal of Organometallic Chemistry, 2013, vol. 743, p. 44 - 48


1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Xilinglab Co., Ltd. | +8618381337976 | bd@xilinglab.com | China | 86 | 58 |
| Capot Chemical Co.,Ltd. | +8613336195806 | sales@capot.com | China | 29731 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21622 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29855 | 58 |
| Changzhou Ansciep Chemical Co., Ltd. | +86 519 86305871 | sales@ansciepchem.com | CHINA | 4241 | 58 |
| Accela ChemBio Inc. | +1-858-6993322 | info@accelachem.com | United States | 21193 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49733 | 58 |
| Richest Group Ltd | 18017061086 | oled@richest-group.com | CHINA | 5600 | 58 |
View Lastest Price from 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-04 | 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
141556-45-8
|
US $0.00-0.00 / KG | 1KG | 98% | 1Ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2022-09-27 | 1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLIUM CHLORIDE
141556-45-8
|
US $0.00 / kg | 1kg | 99% | 1000kg | henan kanbei chemical co.,ltd | |
 |
2021-07-02 | 1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLIUM CHLORIDE
141556-45-8
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- 1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride
141556-45-8
- US $0.00-0.00 / KG
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- 1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLIUM CHLORIDE
141556-45-8
- US $0.00 / kg
- 99%
- henan kanbei chemical co.,ltd
-

- 1,3-BIS(2,4,6-TRIMETHYLPHENYL)IMIDAZOLIUM CHLORIDE
141556-45-8
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
141556-45-8(1,3-Bis(2,4,6-trimethylphenyl)imidazolium chloride )Related Search:
1of2






