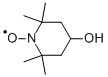
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy
- CAS No.
- 2226-96-2
- Chemical Name:
- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy
- Synonyms
- TEMPOL;4-OH-TEMPO;4-HYDROXY-TEMPO;Inhibitor 701;ZJ-701;TMPN;HYDROXY-TEMPO;Polymerization inhibitor 701;Tetramethylpiperidinol N-oxyl;4-hydroxy-2,2,6,6-tetramethylpiperidinoxyl
- CBNumber:
- CB5782374
- Molecular Formula:
- C9H18NO2*
- Molecular Weight:
- 172.24
- MDL Number:
- MFCD00006478
- MOL File:
- 2226-96-2.mol
- MSDS File:
- SDS
| Product description | Number | Pack Size | Price |
| 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (free radical) for synthesis | 8.40130 | 25G | $89.8 |
| 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (free radical) for synthesis | 8.40130 | 100G | $257 |
| 4-Hydroxy-TEMPO 97% | 176141 | 1g | $35.3 |
| 4-Hydroxy-TEMPO 97% | 176141 | 5g | $101 |
| 4-Hydroxy-2,2,6,6-tetramethylpiperidine 1-Oxyl Free Radical >98.0%(GC) | H0865 | 5g | $57 |
| More product size | |||
| Melting point | 69-71 °C(lit.) |
|---|---|
| Boiling point | 302.58°C (rough estimate) |
| Density | 1.0402 (rough estimate) |
| vapor pressure | 0.025Pa at 20℃ |
| refractive index | 1.4350 (estimate) |
| Flash point | 146°(295°F) |
| storage temp. | 2-8°C |
| solubility | 1670g/l |
| form | Crystals or Crystalline Powder |
| pka | 5.07[at 20 ℃] |
| color | Orange |
| PH | 8.2 (20g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Merck | 14,9141 |
| BRN | 1422990 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Cosmetics Ingredients Functions | ANTIOXIDANT |
| InChIKey | FAPCFNWEPHTUQK-UHFFFAOYSA-N |
| LogP | 0.56 at 25℃ |
| CAS DataBase Reference | 2226-96-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | U78ZX2F65X |
| NCI Drug Dictionary | Tempol |
| NIST Chemistry Reference | 1-Piperidinyloxy, 4-hydroxy-2,2,6,6-tetramethyl-(2226-96-2) |
| EPA Substance Registry System | 1-Piperidinyloxy, 4-hydroxy-2,2,6,6-tetramethyl- (2226-96-2) |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H318 | |||||||||
| Precautionary statements | P264-P270-P280-P301+P312-P305+P351+P338-P501 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-36/38 | |||||||||
| Safety Statements | 26-36-37/39-36/37/39-27 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TN8991000 | |||||||||
| Autoignition Temperature | 260°C (DIN 51794) | |||||||||
| TSCA | TSCA listed | |||||||||
| HS Code | 29333999 | |||||||||
| Hazardous Substances Data | 2226-96-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral in rat: 1053mg/kg | |||||||||
| NFPA 704 |
|
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy price More Price(63)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.40130 | 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (free radical) for synthesis | 2226-96-2 | 25G | $89.8 | 2025-07-31 | Buy |
| Sigma-Aldrich | 8.40130 | 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (free radical) for synthesis | 2226-96-2 | 100G | $257 | 2025-07-31 | Buy |
| Sigma-Aldrich | 176141 | 4-Hydroxy-TEMPO 97% | 2226-96-2 | 1g | $35.3 | 2025-07-31 | Buy |
| Sigma-Aldrich | 176141 | 4-Hydroxy-TEMPO 97% | 2226-96-2 | 5g | $101 | 2025-07-31 | Buy |
| TCI Chemical | H0865 | 4-Hydroxy-2,2,6,6-tetramethylpiperidine 1-Oxyl Free Radical >98.0%(GC) | 2226-96-2 | 5g | $57 | 2025-07-31 | Buy |
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy Chemical Properties,Uses,Production
Description
TEMPOL is a piperidine nitroxide and spin label with superoxide dismutase (SOD) mimetic activity. It inhibits lipid peroxidation in rat liver microsomes with 50% inhibition of microsomal lipid peroxidation (IP50) values of 117, 61, and 381 μM for peroxidation induced by iron plus NADPH, iron plus ascorbate, and t-butylhydroperoxide, respectively. TEMPOL (1 mM) inhibits production of superoxide anions by 92% via a xanthine-xanthine oxidase reaction in vitro. It reduces mean arterial pressure and heart rate in spontaneously hypertensive rats (ED50s = 70 and 63 μmol/kg, respectively) when administered intravenously. TEMPOL is a cell-permeable spin label that has been used to quantify intracellular oxygen in various cell types by electron spin resonance (ESR) spectroscopy.
Chemical Properties
solid
Uses
A free radical scavenger
Uses
Spin label for EPR studies; phase transfer dehydration catalyst; antioxidant; inhibitor of olefin free radical polymerization.
Uses
Tempol, a water-soluble piperidine nitroxide derivative having nonspecific radical-scavenging and superoxide dismutase (SOD) activity, protects cultured aerobic, but not hypoxic, cells against radiation-induced killing. Protection does not depend on intracellular thiols and does not involve O2-depletion. Tempol reacts with peroxyl radicals and can also oxidize DNA-bound metal ions, thereby interfering with OH? generation.
Application
In biochemical research, 4-hydroxy-TEMPO has been investigated as an agent for limiting reactive oxygen species. It catalyzes the disproportionation of superoxide, facilitates hydrogen peroxide metabolism, and inhibits Fenton chemistry.4-Hydroxy-TEMPO, along with related nitroxides, are being studied for their potential antioxidant properties.On an industrial-scale 4-hydroxy-TEMPO is often present as a structural element in hindered amine light stabilizers, which are commonly used stabilizers in plastics, it is also used as a polymerisation inhibitor, particularly during the purification of styrene.
Synthesis Reference(s)
Synthetic Communications, 19, p. 3509, 1989 DOI: 10.1080/00397918908052760
General Description
4-Hydroxy-TEMPO is a 4-substituted 2,2,6,6-tetramethylpiperidyl-1-oxy (TEMPO) derivative. It is a low-molecular weight compound and has been proposed as superoxide dismutase mimic.
Flammability and Explosibility
Non flammable
Biological Activity
Superoxide scavenger that displays neuroprotective, anti-inflammatory and analgesic effects.
storage
Store at -20°C
References
[1] TATIANA LIPMAN Philip L Rinat Tabakman. Neuroprotective effects of the stable nitroxide compound Tempol on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in the Nerve Growth Factor-differentiated model of pheochromocytoma PC12 cells[J]. European journal of pharmacology, 2006, 549 1: Pages 50-57. DOI:10.1016/j.ejphar.2006.08.022
[2] GREGOR S GURON. Acute effects of the superoxide dismutase mimetic tempol on split kidney function in two-kidney one-clip hypertensive rats.[J]. Journal of Hypertension, 2006, 24 2: 387-394. DOI:10.1097/01.hjh.0000200511.02700.99
[3] AYELET M. SAMUNI . γ-Irradiation Damage to Liposomes Differing in Composition and Their Protection by Nitroxides[J]. Free Radical Biology and Medicine, 1997, 23 7: Pages 972-979. DOI:10.1016/s0891-5849(97)00123-8
[4] CATIA C F BERNARDY. Tempol, a Superoxide Dismutase Mimetic Agent, Inhibits Superoxide Anion-Induced Inflammatory Pain in Mice.[J]. BioMed Research International, 2017: 9584819. DOI:10.1155/2017/9584819
[5] MILES J. DE BLASIO . The superoxide dismutase mimetic tempol blunts diabetes-induced upregulation of NADPH oxidase and endoplasmic reticulum stress in a rat model of diabetic nephropathy[J]. European journal of pharmacology, 2017, 807: Pages 12-20. DOI:10.1016/j.ejphar.2017.04.026
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| HANGZHOU HAILAN CHEMICAL CO.,LTD. | +86-57156122185 +86-15967590394 | sales@hailan-chem.com | China | 393 | 58 |
| Jiangxi Lotchem Co.,Ltd. | +8615270827708 | lancetu@jxlotchem.com | CHINA | 338 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2950 | 58 |
| ZHEJIANG ESTCHEM CO.,LTD | 15957180504 | sales@zjestchem.com | China | 193 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615350571055 | Sibel@chuanghaibio.com | China | 8754 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5242 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +8617736087130 | catherine@yjchem.com.cn | China | 994 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +86-15350851019; +8615350851019 | admin@86-ss.com | China | 999 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 | niki@zlchemi.com | China | 7245 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718; +8613336195806 | sales@capot.com | China | 29735 | 60 |
Related articles
- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy: A Promising Radical Scavenger for Preventing Radiation-Induced Alopecia
- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy is a radical scavenger with therapeutic potential in preventing radiation-induced ....
- Jan 4,2024
- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy: pharmacokinetics and activity
- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy is a heterocyclic compound with diverse potential effects, including antioxidative....
- Jul 11,2023
- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy- Reaction / Application on synthetic works
- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy is a heterocyclic compound, which is a 4-substituted 2,2,6,6-tetramethylpiperidyl-....
- Nov 8,2019
View Lastest Price from 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2026-01-08 | Tempol
2226-96-2
|
US $32.00 / mL | 99.98% | 10g | TargetMol Chemicals Inc. | ||
 |
2026-01-08 | 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy
2226-96-2
|
0.99 | RongNa Biotechnology Co.,Ltd | ||||
 |
2026-01-08 | 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy
2226-96-2
|
US $3.00 / kg | 1 mkg | 99.5% | 500mt | Jinan Finer Chemical Co., Ltd |
-

- Tempol
2226-96-2
- US $32.00 / mL
- 99.98%
- TargetMol Chemicals Inc.
-

- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy
2226-96-2
- 0.99
- RongNa Biotechnology Co.,Ltd
-

- 4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy
2226-96-2
- US $3.00 / kg
- 99.5%
- Jinan Finer Chemical Co., Ltd
2226-96-2(4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy)Related Search:
1of4