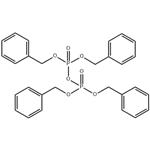
Tetrabenzyl pyrophosphate
- CAS No.
- 990-91-0
- Chemical Name:
- Tetrabenzyl pyrophosphate
- Synonyms
- PYROPHOSPHORIC ACID TETRABENZYL ESTER;TETRABENZYL DIPHOSPHATE;C2gH2gO7P2;TetrabenzyL;Bis(phenylmethox;Benzyl Pyrophosphate;Tetracyl pyrophosphate;Tetrabenzyl yrophosphate;Fosaprepitant Impurity S;Tetrabenzyl Pyrophosphte
- CBNumber:
- CB9300303
- Molecular Formula:
- C28H28O7P2
- Molecular Weight:
- 538.47
- MDL Number:
- MFCD00051941
- MOL File:
- 990-91-0.mol
- MSDS File:
- SDS
| Product description | Number | Pack Size | Price |
| Tetrabenzyl pyrophosphate 98% | 418633 | 1g | $333 |
| Tetrabenzyl Pyrophosphate >98.0%(HPLC) | P1223 | 1g | $70 |
| Tetrabenzyl pyrophosphate | 289972 | 100mg | $346 |
| Tetrabenzyl pyrophosphate | T289450 | 100g | $545 |
| Tetrabenzyl diphosphate 95+% | 090008 | 25g | $288 |
| More product size | |||
| Melting point | 63-66 °C (lit.) |
|---|---|
| Boiling point | 601.6±55.0 °C(Predicted) |
| Density | 1.289±0.06 g/cm3(Predicted) |
| vapor pressure | 0.001Pa at 140℃ |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Powder or Crystalline Powder |
| color | White to off-white |
| Water Solubility | Slightly soluble in water. |
| Sensitive | Moisture Sensitive |
| BRN | 2068292 |
| InChI | InChI=1S/C28H28O7P2/c29-36(31-21-25-13-5-1-6-14-25,32-22-26-15-7-2-8-16-26)35-37(30,33-23-27-17-9-3-10-18-27)34-24-28-19-11-4-12-20-28/h1-20H,21-24H2 |
| InChIKey | NSBNXCZCLRBQTA-UHFFFAOYSA-N |
| SMILES | P(=O)(OCC1C=CC=CC=1)(OCC1C=CC=CC=1)OP(=O)(OCC1C=CC=CC=1)OCC1C=CC=CC=1 |
| CAS DataBase Reference | 990-91-0(CAS DataBase Reference) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 3261 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10-21 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29209090 | |||||||||
| Limited Quantities | 1.0 L (0.3 gallons) (liquid) or 1 Kg (2.2 lbs) (solid) | |||||||||
| Excepted Quantities | Max Inner Pack (30g or 30ml) and Max Outer Pack (500g or 500ml) | |||||||||
| NFPA 704 |
|
Tetrabenzyl pyrophosphate price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 418633 | Tetrabenzyl pyrophosphate 98% | 990-91-0 | 1g | $333 | 2025-07-31 | Buy |
| TCI Chemical | P1223 | Tetrabenzyl Pyrophosphate >98.0%(HPLC) | 990-91-0 | 1g | $70 | 2025-07-31 | Buy |
| Usbiological | 289972 | Tetrabenzyl pyrophosphate | 990-91-0 | 100mg | $346 | 2021-12-16 | Buy |
| TRC | T289450 | Tetrabenzyl pyrophosphate | 990-91-0 | 100g | $545 | 2021-12-16 | Buy |
| Matrix Scientific | 090008 | Tetrabenzyl diphosphate 95+% | 990-91-0 | 25g | $288 | 2021-12-16 | Buy |
Tetrabenzyl pyrophosphate Chemical Properties,Uses,Production
Chemical Properties
White Solid
Characteristics
Tetrabenzyl pyrophosphate and diphenylphosphinic anhydride, with two phosphoryl groups (P O) as ligating sites, can be used as novel ionophores to make Pb2+-selective membrane electrodes. A good result was obtained with tetrabenzyl pyrophosphate, and the electrode based on this ionophore and bis(1-butylpentyl) adipate as a solvent mediator in a poly(vinyl chloride) membrane matrix exhibited a near-Nernstian response to Pb2+ in the concentration range of 1×105–1×102 M with a slope of 28.7 mV per concentration decade in a solution containing 0.1 M Mg(NO3)2. Tetrabenzyl pyrophosphate showed the best response to Pb2+, almost free from interference by other metal cations. By adding the ionic additive potassium tetrakis(p-chlorophenyl)borate (KTpClPB; 40 mol% relative to tetrabenzyl pyrophosphate), a drastic change occurred in the electrode response, showing a monovalent species probably due to the formation of PbA+, where A stands for anions present in the sample solution, and decreased the selectivity of the electrode to other metal cations[1].
Uses
Phosphorylating reagent.
Uses
Tetrabenzyl pyrophosphate is used in the preparation of phosphoryl derivatives of shikimic acid in the presence of LDA. It is used in the preparation of dibenzyl phosphoro fluoridate in the presence of cesium fluoride as a catalyst. It is also used as a precursor in pharmaceuticals and involved in the phosphorylation of inositol derivatives.
Preparation
The production of tetrabenzyl pyrophosphate by the action of acyl chlorides on dibenzyl hydrogen phosphate, and a novel reaction of tetraphenyl pyrophosphate.
Preparation of Tetrabenzyl Pyrophosphate: A flask fitted with a nitrogen inlet, overhead stirrer, teflon-coated thermocouple probe, and pressure-equalizing addition funnel was charged with 350 milliliters of dry (water content ≦50 μg/mL), peroxide-free tetrahydrofuran, followed by 50.0 grams (174 millimoles) of dibenzylphosphoric acid (DBP), and the resulting mixture was stirred until the solid dissolved (about 10-15 minutes). A solution of 18.9 grams (91.6 millimoles) of dicyclohexylcarbodiimide (DCC) in 215 milliliters of THF was added from the addition funnel to a stirred, cooled (water-bath) solution of DBP at a rate to maintain the temperature at about 20°-25° C. The reaction is slightly exothermic and the addition took about 30 minutes. Within minutes, a precipitate of dicyclohexylurea formed in the mixture. Stirring was continued for about 2 hours at 20°-25° C. The reaction was monitored by HPLC assay using VYDAC C-18 (300A, 4.6×250 mm) column with water (0.02M KH2PO4) acetonitrile as eluent. The reaction was complete in about 1 hour (assay showing <2 percent unreacted DBP). The mixture was then filtered while excluding moisture to remove dicyclohexylurea. The filter cake was washed with two 25 milliliter portions of THF. The solution when assayed by HPLC showed 45.9 grams (98 percent) yield of tetrabenzyl pyrophosphate (TBPP) in 620 milliliters of THF (0.137M). The filtrate was then stored at 0° C. with exclusion of moisture until the next step.
Synthesis

1623-08-1

990-91-0
An overhead stirrer, thermocouple, N2 inlet and charging funnel were assembled in a 12L round bottom flask. Dibenzyl phosphate (762 g) and isopropyl acetate (3 L) were added to the reaction flask. The mixture was cooled to 3 ± 3 °C, followed by the slow addition of 1.08 M solution of dicyclohexylcarbodiimide (DCC) (1.30 L) through the addition funnel while the reaction temperature was controlled to maintain at 3 ± 3 °C. The addition time was usually 25-35 min and the reaction was usually completed within 30 min. Upon completion of the reaction, the cold slurry was filtered and the dicyclohexylurea filter cake was washed with isopropyl acetate (3 x 600 mL). The filtrate and washings were combined and concentrated under vacuum to a final volume of 1.5 L. The concentrate was transferred to another 12 L round-bottomed flask, which was also equipped with an overhead stirrer, a thermocouple, an N2 inlet, and a charging funnel. The concentrate was diluted with heptane (500 mL) and 1 mol% of tetrabenzyl pyrophosphate (8 g) was added as a crystal seed to promote crystallization. Subsequently, heptane (4.0 L) was slowly added to the stirring slurry over 30 min at room temperature. The mixture was cooled to 3 ± 3 °C and aged for 1 hour. The slurry was filtered and the filter cake was washed with 20% isopropyl acetate/heptane solution (3 x 500 mL). The product filter cake was dried under vacuum and under nitrogen protection at room temperature overnight. The final product was obtained as tetrabenzyl pyrophosphate (671 g, 1.25 mol, corrected for crystalline species) as a white crystalline solid in 91% regulated yield and the product was stored in a refrigerator.
References
[1] Dafeng Xu, Takashi Katsu. “Tetrabenzyl pyrophosphate as a new class of neutral carrier responsive to lead ion.” Talanta 51 2 (2000): Pages 365-371.
Tetrabenzyl pyrophosphate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TianRui Pharmaceutical Chemical Co., Ltd. | +86-0551-68665055 +86-+86-18616906106 | sales@trywchem.com | China | 186 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615350571055 | Sibel@chuanghaibio.com | China | 8753 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5242 | 58 |
| ZHENGZHOU JIUYI TIME NEW MATERIALS CO,.LTD | +86-13017695106 +86-13676922317 | jiuyitime@fdachem.com | China | 16513 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 880 | 50 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718; +8613336195806 | sales@capot.com | China | 29735 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21597 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| Zjartschem | +86-571 87238903 | jocelynpan@zjarts.com | CHINA | 988 | 58 |
Related articles
- Application research of tetrabenzyl pyrophosphate
- Tetrabenzyl pyrophosphate has been used to synthesize phosphate-containing biologically active molecules and prodrugs. Its app....
- Oct 11,2025
View Lastest Price from Tetrabenzyl pyrophosphate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2026-02-22 | Tetrabenzyl pyrophosphate
990-91-0
|
0.99 | RongNa Biotechnology Co.,Ltd | ||||
 |
2026-01-30 | TETRABENZYL DIPHOSPHATE
990-91-0
|
US $1.00 / kg | 1kg | 99% | 10 mt | Hebei Chuanghai Biotechnology Co., Ltd | |
 |
2025-12-01 | Tetrabenzyl pyrophosphate
990-91-0
|
US $0.00 / kg | 1kg | 99% | 10000KGS | Shaanxi Dideu New Materials Co. Ltd |
-

- Tetrabenzyl pyrophosphate
990-91-0
- 0.99
- RongNa Biotechnology Co.,Ltd
-

- TETRABENZYL DIPHOSPHATE
990-91-0
- US $1.00 / kg
- 99%
- Hebei Chuanghai Biotechnology Co., Ltd
-

- Tetrabenzyl pyrophosphate
990-91-0
- US $0.00 / kg
- 99%
- Shaanxi Dideu New Materials Co. Ltd





