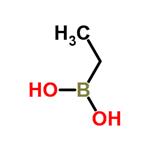
- Ethylboronic acid
-
- $3.00 / 25KG
-
2025-10-13
- CAS:4433-63-0
- Min. Order: 0.1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Ethylboronic acid
-

- $0.00 / 1KG
-
2025-04-04
- CAS:4433-63-0
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton
- Ethylboronic acid
-

- $15.00 / 1KG
-
2021-08-12
- CAS:4433-63-0
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Ethylboronic acid Basic information |
| | Ethylboronic acid Chemical Properties |
| Melting point | 161-162°C | | Boiling point | 154.0±23.0 °C(Predicted) | | density | 0.941±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | solubility | DMSO (Slightly), Water (Slightly) | | pka | 10.18±0.43(Predicted) | | form | Crystalline Powder, Needles or Flakes | | color | White to slightly yellow | | Sensitive | Hygroscopic | | BRN | 1731546 | | InChI | InChI=1S/C2H7BO2/c1-2-3(4)5/h4-5H,2H2,1H3 | | InChIKey | PAVZHTXVORCEHP-UHFFFAOYSA-N | | SMILES | C(B(O)O)C | | CAS DataBase Reference | 4433-63-0(CAS DataBase Reference) | | NIST Chemistry Reference | Ethyldihydroxyborane(4433-63-0) |
| Hazard Codes | Xi,Xn | | Risk Statements | 36/37/38-22 | | Safety Statements | 37/39-26-36 | | WGK Germany | WGK 3 | | TSCA | No | | HazardClass | IRRITANT, KEEP COLD | | HS Code | 29319090 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| | Ethylboronic acid Usage And Synthesis |
| Chemical Properties | white to slightly yellow crystalline powder, | | Uses | Ethylboronic Acid is the sole precursor in the successful preparation of boron-doped graphene (B-doped graphene) films with large area. | | Synthesis | Alkylboronic acid pinacol ester (2 mmol) and methanol (10 mL) were charged into a 10 mL glass vial with a magnetic stirring bar, potassium hydrofluoride (2 mL, 4.5 M saturated aqueous solution) was added slowly and dropwise to the mixture, and the mixture was stirred for 1 hour at room temperature. All volatile compounds were then removed under vacuum, and the organic residue was redissolved in methanol (6 mL), followed by the addition of water (6-10 mL) and evaporation of all volatile compounds (this process was performed 3 times). The solid residue was stirred with dry acetone (8 mL), then the liquid phase was carefully decanted and the residual inorganic salts were washed with more acetone (3 2 mL), the filtrate was collected, and the filtrate was concentrated in vacuo to give the potassium ethyltrifluoroborate salt intermediate. Potassium alkyltrifluoroborate (0.4 mmol) and water (1 mL) were injected into a 10 mL glass vial fitted with a magnetic stirring bar, then silica (100 mg) was added to the vial, and the mixture was stirred for 1-2 hr at room temperature, and the progress of the reaction was monitored by 11B-NMR, and when the reaction had proceeded to its full extent, a mixture of ethyl acetate and ether (10 mL) was used to The mixture was diluted and the organic phase was separated by filtering the mixture through diatomaceous earth. The aqueous phase was extracted with a mixed solution of ethyl acetate and ether, and the combined organic layers were dried with anhydrous sodium sulfate, and finally the target product was obtained by removing the solvent in vacuum. |
| | Ethylboronic acid Preparation Products And Raw materials |
|