Fluzoparib manufacturers
- Fluzoparib
-

- $55.00 / 1mg
-
2026-02-02
- CAS:1358715-18-0
- Min. Order:
- Purity: 99.63%
- Supply Ability: 10g
|
| | Fluzoparib Basic information |
| Product Name: | Fluzoparib | | Synonyms: | 1(2H)-Phthalazinone, 4-[[3-[[5,6-dihydro-2-(trifluoromethyl)[1,2,4]triazolo[1,5-a]pyrazin-7(8H)-yl]carbonyl]-4-fluorophenyl]methyl]-;Fuzuopali;Fuzuloparib;HS10160;Fluzoparib;SHR 3162,homologous recombination repair,Inhibitor,poly ADP ribose polymerase,Fluzoparib,inhibit,ovarian,PARP,PARP1,SHR-3162,Fuzuloparib,cancer;Sulfamethoxazole Impurity 21;Fluzoparib, 10 mM in DMSO | | CAS: | 1358715-18-0 | | MF: | C22H16F4N6O2 | | MW: | 472.4 | | EINECS: | | | Product Categories: | | | Mol File: | 1358715-18-0.mol | 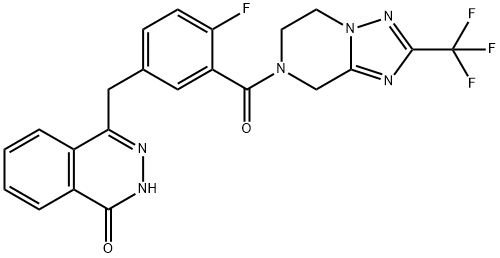 |
| | Fluzoparib Chemical Properties |
| density | 1.59±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMSO : 50 mg/mL (105.84 mM; Need ultrasonic) | | pka | 12.06±0.40(Predicted) | | form | Solid | | color | White to off-white | | InChI | InChI=1S/C22H16F4N6O2/c23-16-6-5-12(10-17-13-3-1-2-4-14(13)19(33)29-28-17)9-15(16)20(34)31-7-8-32-18(11-31)27-21(30-32)22(24,25)26/h1-6,9H,7-8,10-11H2,(H,29,33) | | InChIKey | XJGXCBHXFWBOTN-UHFFFAOYSA-N | | SMILES | C1(=O)C2=C(C=CC=C2)C(CC2=CC=C(F)C(C(N3CCN4N=C(C(F)(F)F)N=C4C3)=O)=C2)=NN1 |
| | Fluzoparib Usage And Synthesis |
| Uses | Fluzoparib (SHR3162) is a potent and orally active PARP1 inhibitor (IC50=1.46±0.72 nM, a cell-free enzymatic assay) with superior antitumor activity. Fluzoparib selectively inhibits the proliferation of homologous recombination repair (HR)-deficient cells, and sensitizes both HR-deficient and HR-proficient cells to cytotoxic agents. Fluzoparib exhibits good pharmacokinetic properties in vivo and can be used for BRCA1/2-mutant relapsed ovarian cancer research[1]. | | Description | Fuzuloparib is mainly used to treat patients with recurrent ovarian, fallopian tube or primary peritoneal cancer that is sensitive to platinum-based drugs, especially those with germline breast cancer oncogene (BRCA) mutations. Tumor cells with BRCA mutations are particularly sensitive to PARP inhibitors due to defects in the homologous recombination (HR) repair pathway. | | Mechanism of action | Fuzuloparib is an orally active poly(ADP-ribose) polymerase (PARP) inhibitor. PARP plays an important role in the cell's DNA damage response (DDR) and promotes the recruitment of DNA repair mechanisms by catalyzing PARylation. Fuzuloparib inhibits the function of PARP, leading to the accumulation of DNA double-strand breaks (DSBs) in tumor cells, ultimately achieving synthetic lethality. | | Synthesis | The synthesis started with 2-(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyrazole, which was first reduced to the tetrahydropyrazole and then converted to the hydrochloride salt 26.2. Fuzuloparib (26) was prepared by HATU-mediated amidation of 26.2 with benzoic acid 26.3 in 88% yield.
 | | in vivo | Fluzoparib (oral gavage; 0.3, 1, or 3 mg/kg; single dose) exhibits a good pharmacokinetic profile in Female Balb/cA nude mice (5-6 weeks old) mice bearing MDA-MB-436. After a single oral dose, fluzoparib is rapidly absorbed and rapidly cleared from blood at all dose levels; plasma concentrations of fluzoparib quickly reaches maximum within 2 hours. In contrast, concentrations of fluzoparib in tumor remains at high levels even at 24 hours after dosing (57.9 ng/g , 39.3 ng/g, and 85.6 ng/g for doses of 0.3, 1, and 3 mg/kg, respectively)[1].Fluzoparib (oral gavage; 30 mg/kg; 21 days) apparently inhibits the growth of tumor with an inhibition rate of 59% (day 21) at 30 mg/kg, and it does not cause significant loss of body weight in Nude mice bearing MDA-MB-436 (BRCA1-deficient) model[1].Fluzoparib (3mg/kg) combines with Cisplatin, Paclitaxel, or Apatinib (oral gavage; BID; 21 days) causes growth inhibition with rates of 61.4%, 55.3%, and 72.8%, respectively.Fluzoparib, Cisplatin, and Apatinib combination or Fluzoparib, Paclitaxel, and Apatinib combination can cause growth inhibition with rates of 84.9% and 75.6% (day 21), respectively in vivo.The 2-drug combination of Fluzoparib with cisplatin and The 3-drug Fluzoparib, Cisplatin, and Apatinib combination lead to loss of body weight, whereas no apparent toxicity was observed in other combinations[1]. | | IC 50 | PARP-1: 1.46±0.72? nM (IC50) | | References | [1] Lei Wang, et al. Pharmacologic characterization of fluzoparib, a novel poly(ADP-ribose) polymerase inhibitor undergoing clinical trials. Cancer Sci. 2019 Mar;110(3):1064-1075. DOI:10.1111/cas.13947
[2] Huiping Li, et al. Phase I dose-escalation and expansion study of PARP inhibitor, fluzoparib (SHR3162), in patients with advanced solid tumors. Chin J Cancer Res. 2020 Jun;32(3):370-382. DOI:10.21147/j.issn.1000-9604.2020.03.08 |
| | Fluzoparib Preparation Products And Raw materials |
| Raw materials | 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)Methyl)benzoic acid-->5-Bromo-2-fluorobenzoic acid-->2-(TRIFLUOROMETHYL)-[1,2,4]TRIAZOLO[1,5-A]PYRAZINE-->2-(TRIFLUOROMETHYL)-5,6,7,8-TETRAHYDRO-[1,2,4]TRIAZOLO[1,5-A]PYRAZINE |
|