|
|
| | 7-Chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid Basic information |
| Product Name: | 7-Chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid | | Synonyms: | 7-Chloro-1-Cyclopropyl-6-Fluoro-4-Oxo-1,4-Dihydro-1,8-Naphthyridin-3-Carboxylic Acid;1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyl ridine carbox.acid;7-Chloro-1-Cyclopropyl-6-Fluoro-1,4-Dihydro-4-Qxoquinoline-3-CarboxylicAcid98%;7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylicacid;7-Chloro-1-cyclopropyl-6-fluor;7-Chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-Ox;7-Chloro-1-cyclopropyl-1,4-dihydro-3-oxo-1,8-naphthyridine-3-carboxylic aci;1-CYCLOPROPYL-6-FLUORO-1,4-DIHYDRO-4-OXO-1,8-NAPHTHYLRIDINE CARBOXYLIC ACID 99% | | CAS: | 100361-18-0 | | MF: | C12H8ClFN2O3 | | MW: | 282.65 | | EINECS: | 600-082-3 | | Product Categories: | Fluorine series;Heterocycles;Intermediate;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 100361-18-0.mol | 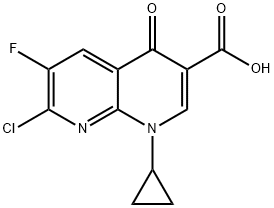 |
| | 7-Chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid Chemical Properties |
| Melting point | 209-212 °C | | Boiling point | 485.9±45.0 °C(Predicted) | | density | 1.727±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | pka | 5.96±0.70(Predicted) | | form | powder to crystal | | color | White to Almost white | | CAS DataBase Reference | 100361-18-0 |
| | 7-Chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid Usage And Synthesis |
| Chemical Properties | Off-white or light yellow powder | | Uses | Gemifloxacin intermediate as antibacterial agent. | | Synthesis | Ethyl 7-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylate (0.1 g, 0.32 mmol) was used as a raw material and dissolved in THF (5 ml). Concentrated HCl (45 μL, 0.7 mmol) was added to this solution. The reaction mixture was heated to reflux and the reaction was continued for 2 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the completion of the reaction, the reaction mixture was cooled to room temperature. The precipitated solid was collected by filtration to afford the target product 7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid in a yield of 70 mg with 78% yield. | | References | [1] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 7, p. 2428 - 2433
[2] Journal of Medicinal Chemistry, 2002, vol. 45, # 25, p. 5564 - 5575
[3] Patent: WO2005/40164, 2005, A1. Location in patent: Page/Page column 10-12 |
| | 7-Chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid Preparation Products And Raw materials |
|