|
|
| | Ethyl 3,4-bis(2-methoxyethoxy)benzoate Basic information |
| Product Name: | Ethyl 3,4-bis(2-methoxyethoxy)benzoate | | Synonyms: | 3,4-Bis(2-methoxyethoxy)benzoic acid ethyl ester;Ethyl 3,4-bis(2-methoxyethoxy)benzoate;Thyl3,4-bis(2-methoxyethoxy)benzoate;Erlotinib interMediate II;Benzoic acid,3,4-bis(2-Methoxyethoxy)-, ethyl ester;3,4-bis(2-methoxyethoxy)benzoate;Ethyl 3,4-bis(2-methoxyethoxy);Erlotinib Hydrochloride iMpurity 30 | | CAS: | 183322-16-9 | | MF: | C15H22O6 | | MW: | 298.33 | | EINECS: | 605-991-9 | | Product Categories: | Erlotinib | | Mol File: | 183322-16-9.mol | 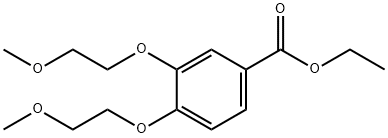 |
| | Ethyl 3,4-bis(2-methoxyethoxy)benzoate Chemical Properties |
| Melting point | 55-58 °C | | Boiling point | 401.7±45.0 °C(Predicted) | | density | 1.102 | | storage temp. | Sealed in dry,Room Temperature | | form | powder to crystal | | color | White to Almost white | | InChI | InChI=1S/C15H22O6/c1-4-19-15(16)12-5-6-13(20-9-7-17-2)14(11-12)21-10-8-18-3/h5-6,11H,4,7-10H2,1-3H3 | | InChIKey | VGFZRAVMWXHEJB-UHFFFAOYSA-N | | SMILES | C(OCC)(=O)C1=CC=C(OCCOC)C(OCCOC)=C1 |
| | Ethyl 3,4-bis(2-methoxyethoxy)benzoate Usage And Synthesis |
| Uses | 3,4-bis(2-Methoxyethoxy)benzoic Acid Ethyl Ester is a compound used in the synthesis of Erlotinib (E625008), a cancer treatment medicine. | | Synthesis | (3) Preparation of ethyl 3,4-bis(2-methoxyethoxy)benzoate 13: Ethyl 3,4-dihydroxybenzoate 12 (5 g, 27.45 mmol) was placed in a 250 mL two-necked flask and acetone (100 mL), potassium carbonate (9.48 g, 68.63 mmol), potassium iodide (0.5 g) and 2-bromoethyl methyl ether ( 7.84 mL, 82.35 mmol). The reaction mixture was heated to reflux at 60 °C for 19 hours. Upon completion of the reaction, the reaction solution was cooled to 5 °C and stirred for 30 min, followed by filtration and concentration to dryness. The resulting solid was dried by oil pumping for 22 h to give the khaki colored solid product 13 (8.19 g, 100% yield).1H-NMR (CDCl3) data: δ 1.32 (t, 3H, J = 7Hz), 3.41 (s, 6H), 3.76 (m, 4H), 4.15 (m, 4H), 4.29 (q, 2H, J = 7Hz), 6.85 (d, 1H, J = 8.4Hz), 7.53 (dd, 1H, J = 8.4Hz, J = 2.3Hz), 7.63 (dd, 1H, J = 8.4Hz, J = 2.3Hz). | | References | [1] Patent: US2010/267949, 2010, A1. Location in patent: Page/Page column 6
[2] Patent: EP1614676, 2006, A1. Location in patent: Page/Page column 194
[3] Patent: EP2163546, 2016, B1. Location in patent: Paragraph 0125
[4] European Journal of Medicinal Chemistry, 2008, vol. 43, # 7, p. 1478 - 1488
[5] Chemistry - A European Journal, 2015, vol. 21, # 41, p. 14342 - 14346 |
| | Ethyl 3,4-bis(2-methoxyethoxy)benzoate Preparation Products And Raw materials |
|