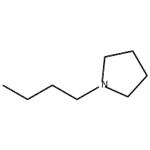
- 1-Butylpyrrolidine
-
- $8.00 / 1kg
-
2025-09-25
- CAS:767-10-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
|
| | 1-Butylpyrrolidine Basic information |
| | 1-Butylpyrrolidine Chemical Properties |
| Boiling point | 155-157 °C/754 mmHg (lit.) | | density | 0.814 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.44(lit.) | | Fp | 97 °F | | storage temp. | Refrigerator, Under inert atmosphere | | solubility | Chloroform (Sparingly), Ethyl Acetate (Slightly) | | pka | 10.62±0.20(Predicted) | | form | Oil | | color | Colourless | | InChI | InChI=1S/C8H17N/c1-2-3-6-9-7-4-5-8-9/h2-8H2,1H3 | | InChIKey | JSHASCFKOSDFHY-UHFFFAOYSA-N | | SMILES | N1(CCCC)CCCC1 | | LogP | 2.209 (est) | | CAS DataBase Reference | 767-10-2(CAS DataBase Reference) | | NIST Chemistry Reference | 1-Butylpyrrolidine(767-10-2) |
| Hazard Codes | T | | Risk Statements | 10-24/25 | | Safety Statements | 16-36/37-45 | | RIDADR | UN 2929 6.1/PG 2 | | WGK Germany | 2 | | RTECS | UX9800000 | | HazardClass | 3.2 | | PackingGroup | III | | Storage Class | 3 - Flammable liquids | | Hazard Classifications | Acute Tox. 3 Dermal
Acute Tox. 3 Oral
Flam. Liq. 3 |
| | 1-Butylpyrrolidine Usage And Synthesis |
| Uses | 1-Butylpyrrolidine can be used in the composition for alcohol recovery solution. | | Uses | 1-Butylpyrrolidine has been used in:
microwave-assisted synthesis of ionic liquid precursor, 1-butyl-1-methylpyrrolidinium methylcarbonate.
[N,N-methylbutylpyrrolidinium] thiosalicylate, ionic liquid.
The reaction of 1-butylpyrrolidine with dimethyl carbonate to yield the ionic liquid precursor, 1-butyl-1-methylpyrrolidinium methylcarbonate, has been investigated under microwave heating conditions and the reaction parameters optimised to achieve 100% yield of the pyrrolidinium salt with no by-products in under 1h. | | Preparation | The synthesis of 1-butylpyrrolidine and its derivatives (1-butylpyrrolidine with a little of 1-butenylpyrrolidines) was developed via a one-pot method from ammonia and 1,4-butandiol. Here, the product of 1-butylpyrrolidine was emphatically investigated, and the yield was 76% under the optimized conditions. Such a route was realized through successive N-alkylation using aqueous ammonia as the nitrogen source over the CuNiPd/ZSM-5 catalyst, which was prepared by a simple incipient wetness method. In this route, 1,4-butandiol not only participated in the formation of the N-heterocycle, but also acted as an alkylating reagent. This work offers a straightforward, economical route for 1-butylpyrrolidine and its derivatives.

One-pot synthesis of 1-butylpyrrolidine and its derivatives from aqueous ammonia and 1,4-butandiol over CuNiPd/ZSM-5 catalysts |
| | 1-Butylpyrrolidine Preparation Products And Raw materials |
|