|
|
| | 2-METHYL-THIOPHENE-3-CARBOXYLIC ACID Basic information |
| Product Name: | 2-METHYL-THIOPHENE-3-CARBOXYLIC ACID | | Synonyms: | 2-METHYL-THIOPHENE-3-CARBOXYLIC ACID;2-Methyl-3-thiophenecarboxylic acid;3-Carboxy-2-methylthiophene;3-Thiophenecarboxylicacid, 2-Methyl-;3-Carboxy-2-methylthiophene, 2-Methyl-3-thenoic acid;2-methylthiophene-3-carboxylicaci | | CAS: | 1918-78-1 | | MF: | C6H6O2S | | MW: | 142.18 | | EINECS: | 200-258-5 | | Product Categories: | | | Mol File: | 1918-78-1.mol | 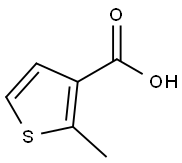 |
| | 2-METHYL-THIOPHENE-3-CARBOXYLIC ACID Chemical Properties |
| Melting point | 106-111 °C | | Boiling point | 271.5±20.0 °C(Predicted) | | density | 1.319±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C(protect from light) | | form | solid | | pka | 4.44±0.20(Predicted) | | color | Brown | | InChI | InChI=1S/C6H6O2S/c1-4-5(6(7)8)2-3-9-4/h2-3H,1H3,(H,7,8) | | InChIKey | YJZOPBSZDIBXBG-UHFFFAOYSA-N | | SMILES | C1(C)SC=CC=1C(O)=O |
| | 2-METHYL-THIOPHENE-3-CARBOXYLIC ACID Usage And Synthesis |
| Synthesis Reference(s) | Tetrahedron Letters, 21, p. 5051, 1980 DOI: 10.1016/S0040-4039(00)71130-0 | | Synthesis | The general procedure for the synthesis of 2-methyl-3-thiophenecarboxylic acid (compound 9) from 3-thiophenecarboxylic acid (compound 8) and iodomethane was carried out as follows: the synthesis was carried out according to the method described in literature [24]. Slowly n-butyllithium (n-BuLi, 1.6 M hexane solution, 1.50 L, 2.40 mol) was added dropwise to a tetrahydrofuran (THF, 2.3 L) solution of diisopropylamine (233 g, 2.30 mol) at 0 °C. After keeping stirring at 0 °C for 40 min, the reaction mixture was cooled to -60 °C and a THF (500 mL) solution of compound 8 (223 g, 1.74 mol) was slowly added and stirring was continued for 1 h at -60 °C. Subsequently, iodomethane (MeI, 254 g, 1.79 mol) was added and the reaction mixture was gradually warmed to room temperature and stirred for 1 hour. After completion of the reaction, the mixture was concentrated under reduced pressure, acidified to pH 1 with aqueous 6N hydrochloric acid and extracted with ethyl acetate (AcOEt). The organic layer was washed with brine and dried over anhydrous sodium sulfate (Na2SO4). After filtration, the solvent was removed by concentration under reduced pressure and the residue was crystallized from water/acetic acid (H2O/AcOH) to give 209 g (85% yield) of compound 9 as a light yellow solid. Its nuclear magnetic resonance hydrogen spectrum (1H NMR, 400 MHz, CDCl3) data were as follows: δ 7.45 (d, J = 5.4 Hz, 1H), 7.01 (d, J = 5.4 Hz, 1H), 2.78 (s, 3H); mass spectrum (MS, ESI) m/z: 143 ([M + H]+), 141 ([M - H]-). | | References | [1] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 18, p. 5417 - 5422
[2] European Journal of Medicinal Chemistry, 2018, vol. 156, p. 269 - 294
[3] European Journal of Medicinal Chemistry, 2015, vol. 102, p. 471 - 476
[4] Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1983, p. 791 - 794
[5] Patent: US6340759, 2002, B1. Location in patent: Example 290 |
| | 2-METHYL-THIOPHENE-3-CARBOXYLIC ACID Preparation Products And Raw materials |
|