- Brivudine
-

- $55.00 / 50mg
-
2026-01-21
- CAS:69304-47-8
- Min. Order:
- Purity: 99.51%
- Supply Ability: 10g
- Brivudine
-

- $55.00 / 50mg
-
2026-01-21
- CAS:69304-47-8
- Min. Order:
- Purity: 99.51%
- Supply Ability: 10g
- Brivudine
-
- $0.00 / 1kg
-
2026-01-04
- CAS:69304-47-8
- Min. Order: 1kg
- Purity: 99.0%
- Supply Ability: 20 tons
|
| | Brivudine Basic information |
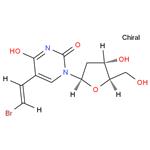
| Product Name: | Brivudine | | Synonyms: | Helpin;Uridine,5-[(1E)-2-broMoethenyl]-2'-deoxy-;(E)-(5)-BROMOVINYL-2-DEOXYURIDINE;(E)-5-(2-Bromovinyl)-2'-deoxyuridine;BVDU;(E)-5-(2-BROMOVINYL)-2'-DEOXY-URIDINE;BRIVUDINE;5-(E)-(2-Bromovinyl)-2-deoxyuridine | | CAS: | 69304-47-8 | | MF: | C11H13BrN2O5 | | MW: | 333.14 | | EINECS: | 1806241-263-5 | | Product Categories: | Inhibitors;API;69304-47-8 | | Mol File: | 69304-47-8.mol |  |
| | Brivudine Chemical Properties |
| Melting point | 165°C | | density | 1.7800 (rough estimate) | | refractive index | 1.6520 (estimate) | | storage temp. | Inert atmosphere,2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 8.37±0.10(Predicted) | | color | Off-White | | Water Solubility | Soluble in water and methanol. | | Merck | 14,1378 | | InChI | 1S/C11H13BrN2O5/c12-2-1-6-4-14(11(18)13-10(6)17)9-3-7(16)8(5-15)19-9/h1-2,4,7-9,15-16H,3,5H2,(H,13,17,18)/b2-1+/t7-,8+,9+/m0/s1 | | InChIKey | ODZBBRURCPAEIQ-CXYVBZRVSA-N | | SMILES | O=C(C(/C=C/Br)=CN1[C@H]2C[C@H](O)[C@@H](CO)O2)NC1=O |
| Safety Statements | 24/25 | | WGK Germany | 3 | | RTECS | YU7355000 | | HS Code | 29349990 |
| | Brivudine Usage And Synthesis |
| Description | (E)-5-(2-Bromovinyl)-2'-deoxyuridine is an thymidine analogue and acts as an anti-viral by inhibiting DNA plymerase. | | Chemical Properties | White solid | | Uses | It is used as pharmaceutical intermediate. | | Uses | Brivudine is a nucloside analog which causes induction of neuronal differentiation in human reporter cell lines and adult stem cells. Anti-Herpes medication. | | Synthesis | GENERAL STEPS: To a solution of (E)-5-(2-carboxyvinyl)-1-(2-deoxy-β-D-erythro-pentofuranosyl)pyrimidine-2,4(1H,3H)-dione (5.777 g, 19.37 mmol) in dimethylformamide (29 mL) was added K2CO3 (5.890 g, 42.61 mmol), and the suspension was stirred at room temperature for for 15 min. A solution of N-bromosuccinimide (3.655 g, 20.53 mmol) was added dropwise over a period of 30 min at 20 °C. After completion of the reaction, the resulting suspension was filtered and the solid was washed with DMF. The combined filtrate and washings were evaporated to dryness under vacuum and the residue was dissolved in methanol. Silica gel was added to this solution, the suspension was evaporated to dryness, and the solid was loaded onto the top of the column. Column chromatography using chloroform/methanol (92:8, v/v) as eluent gave a white solid (5.787 g, 71.9% yield). A white powder was obtained by recrystallization from water. The structure of the product was confirmed by 1H-NMR and 13C-NMR: 1H-NMR (DMSO-d6; 300 MHz) δ 11.59 (1H, bs, NH-3), 8.08 (1H, s, H-6), 7.25 (1H, d, 3J = 13.6 Hz, H-5B), 6.85 (1H, d, J = 13.6 Hz, H-5A) , 6.13 (1H, t, 3J = 6.5 Hz, H-1'), 5.29 (1H, bs, OH-3'), 5.13 (1H, bs, OH-5'), 4.24 (1H, m, H-3'), 3.79 (1H, m, H-4'), 3.66 (2H, m, H-5'), 2.51 (1H, m, H-2'), 2.14 (1H, m, H-2'); 13C-NMR (DMSO-d6; 75 MHz) δ 40.2 (C-2'), 61.3 (C-5'), 70.3 (C-4'), 84.8 (C-3'), 87.8 (C-1'), 108.9 (C-5B), 110.0 (C-5), 130.3 (C-5A), 149.6 , 162.1 (c-2, c-4). | | in vivo | At a dose of 125 mg once daily, brivudine has proved to be superior to aciclovir with respect to reducing the period of new blister production, and has shortened the duration of post-herpetic neuralgia[1]. | | References | [1] Bioorganic and Medicinal Chemistry, 2005, vol. 13, # 24, p. 6663 - 6671
[2] RSC Advances, 2015, vol. 5, # 31, p. 24558 - 24563
[3] Patent: WO2005/12327, 2005, A2. Location in patent: Page/Page column 24-25
[4] Bioorganic and Medicinal Chemistry Letters, 2008, vol. 18, # 20, p. 5640 - 5642
[5] Patent: US6653318, 2003, B1 |
| | Brivudine Preparation Products And Raw materials |
| Raw materials | (E)-5-(2-CARBOXYVINYL)-2'-DEOXYURIDINE-->2,5-Methano-5H,9H-pyrimido[2,1-b][1,5,3]dioxazepin-9-one, 3-[(benzoyloxy)methyl]-8-[(1E)-2-bromoethenyl]-2,3-dihydro-, (2R,3R,5R)--->N-Bromosuccinimide-->Potassium carbonate-->N,N-Dimethylformamide |
|