|
|
| | 4-Bromoisatoic anhydride Basic information |
| Product Name: | 4-Bromoisatoic anhydride | | Synonyms: | 7-Bromo-1H-benzo[d][1,3]oxazine-2,4-dione;7-Bromo-2H-3,1-benzoxazine-2,4(1H)-dione;4-Bromo-isatoic anhydride;4-Bromoisatoic anhydride 97%;7-Bromo-2H-3,1-benzoxazine-2,4(1H)-dione, 7-Bromo-1H-benzo[d][1,3]oxazine-2,4-dione;4-Bromoisatoicanhydride97%;Isatoic anhydride, 4-bromo-;7-bromo-1H-3,1-benzoxazine-2,4-dione | | CAS: | 76561-16-5 | | MF: | C8H4BrNO3 | | MW: | 242.03 | | EINECS: | | | Product Categories: | | | Mol File: | 76561-16-5.mol | 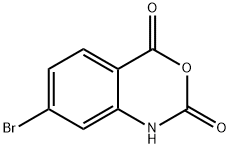 |
| | 4-Bromoisatoic anhydride Chemical Properties |
| density | 1.826 | | storage temp. | 2-8°C | | form | Pale cream, wooly, small crystalline flakes | | pka | 9.95±0.20(Predicted) | | Appearance | Off-white to light yellow Solid |
| | 4-Bromoisatoic anhydride Usage And Synthesis |
| Chemical Properties | White solid | | Synthesis | 7.1: Synthesis of 7-bromo-1H-benzo[d][1,3]oxazine-2,4-dione
In a 250 mL round bottom flask, 5.0 g (23.1 mmol) of 2-amino-4-bromobenzoic acid was dissolved in 50 mL of anhydrous dioxane under nitrogen protection. The reaction mixture was cooled to 0 °C via an ice bath. 2.3 g (7.6 mmol) of triphosgene was added slowly and dropwise. Subsequently, the ice bath was replaced with an oil bath and the reaction mixture was refluxed for 16 hours. After completion of the reaction, it was cooled to room temperature, 100 mL of water was added, and the resulting precipitate was collected by filtration. The precipitate was washed with ether (3 x 25 mL) and then dried in an oven to give 5.6 g (23.1 mmol) of the target product as a beige powder in 100% yield.
1H NMR (DMSO-d6, 300 MHz) δ: 7.29 (d, J = 1.8 Hz, 1H), 7.41 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 7.82 (d, J = 8.4 Hz, 1H), 11.81 (bs, 1H). | | References | [1] Patent: US2011/251194, 2011, A1. Location in patent: Page/Page column 17
[2] Patent: WO2013/147711, 2013, A1. Location in patent: Page/Page column 158
[3] Patent: US2014/371199, 2014, A1. Location in patent: Paragraph 0584
[4] Beilstein Journal of Organic Chemistry, 2018, vol. 14, p. 2529 - 2536
[5] Journal of Medicinal Chemistry, 2015, vol. 58, # 17, p. 6928 - 6937 |
| | 4-Bromoisatoic anhydride Preparation Products And Raw materials |
|