(2S,4R)-4-hydroxy-2-methyl-pyrrolidine-1-carboxylic acid tert-butyl ester manufacturers
|
| | (2S,4R)-4-hydroxy-2-methyl-pyrrolidine-1-carboxylic acid tert-butyl ester Basic information |
| Product Name: | (2S,4R)-4-hydroxy-2-methyl-pyrrolidine-1-carboxylic acid tert-butyl ester | | Synonyms: | (2S,4R)-4-hydroxy-2-methyl-pyrrolidine-1-carboxylic acid tert-butyl ester;tert-butyl (2s,4r)-4-hydroxy-2-methylpyrrolidine-1-carboxylate;(2S,4R)-tert-butyl 4-hydroxy-2-methylpyrrolidine-1-carboxylate;1-Pyrrolidinecarboxylic acid, 4-hydroxy-2-methyl-, 1,1-dimethylethyl ester, (2S,4R)-;1-Pyrrolidinecarboxylic acid, 4-hydroxy-2-methyl-, 1,1-dimet...;(2S,4R)-N-Boc-2-methyl-4-hydroxy-prrolidine;(2S,4R)-1-Boc-4-hydroxy-2-methylpyrrolidine;(2R,4R)-N-Boc-2-methyl-4-hydroxy-pyrrolidine | | CAS: | 114676-61-8 | | MF: | C10H19NO3 | | MW: | 201.26 | | EINECS: | | | Product Categories: | | | Mol File: | 114676-61-8.mol | 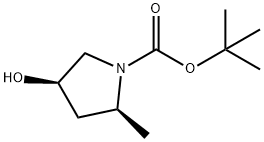 |
| | (2S,4R)-4-hydroxy-2-methyl-pyrrolidine-1-carboxylic acid tert-butyl ester Chemical Properties |
| Melting point | 75-78 °C | | Boiling point | 282.5±33.0 °C(Predicted) | | density | 1.094±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | 14.75±0.40(Predicted) | | Appearance | White to yellow Solid | | Optical Rotation | 20.6°(C=0.01g/100mL, DCM, 20°C, 589nm) | | InChI | InChI=1S/C10H19NO3/c1-7-5-8(12)6-11(7)9(13)14-10(2,3)4/h7-8,12H,5-6H2,1-4H3/t7-,8+/m0/s1 | | InChIKey | BXZADLGAYWRZCR-JGVFFNPUSA-N | | SMILES | N1(C(OC(C)(C)C)=O)C[C@H](O)C[C@@H]1C |
| | (2S,4R)-4-hydroxy-2-methyl-pyrrolidine-1-carboxylic acid tert-butyl ester Usage And Synthesis |
| Synthesis | Tert-butyl (2S,4R)-4-((tert-butyldimethylsilyl)oxy)-2-methylpyrrolidine-1-carboxylate (2.2 g, 6.98 mmol) was dissolved in tetrahydrofuran (30 mL), followed by addition of tetrabutylammonium fluoride (3.65 g, 14.0 mmol). The reaction mixture was stirred at room temperature overnight. After completion of the reaction, the mixture was poured into water (60 mL) and extracted with ethyl acetate (60 mL x 2). The organic phases were combined, washed with saturated sodium chloride solution (60 mL x 2) and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure and the resulting crude product was purified by column chromatography (petroleum ether: ethyl acetate = 2:1) to give 1.47 g of a yellow oily product, i.e. tert-butyl (2S,4R)-4-hydroxy-2-methylpyrrolidine-1-carboxylate in 99% yield. | | References | [1] Patent: EP3401315, 2018, A1. Location in patent: Paragraph 0106; 0111
[2] Journal of Medicinal Chemistry, 1988, vol. 31, # 8, p. 1598 - 1611
[3] Journal of the American Chemical Society, 2008, vol. 130, # 3, p. 875 - 886 |
| | (2S,4R)-4-hydroxy-2-methyl-pyrrolidine-1-carboxylic acid tert-butyl ester Preparation Products And Raw materials |
| Raw materials | 1-Pyrrolidinecarboxylic acid, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methyl-, 1,1-dimethylethyl ester, (2S,4R)--->Tetrahydrofuran-->Tetrabutylammonium fluoride | | Preparation Products | 1-Pyrrolidinecarboxylicacid,4-amino-2-methyl-,1,1-dimethylethylester,(2S-trans)-(9CI) |
|