|
|
| | ETHYL 1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE Basic information |
| Product Name: | ETHYL 1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE | | Synonyms: | Ethyl 1-methyl-3-(trifluoromethyl)pyrazole-4-;Ethyl 1-methyl-3-(trifluoromethyl)-1H-pyrazole-4-carboxylate ,95%;1-Methyl-3-(trifluoromethyl)-1H-pyrazole-4-carboxylic acid ethyl ester;ETHYL 1-METHYL-3-(TRIFLUOROMETHYL)PYRAZOLE-4-CARBOXYLATE;ETHYL 1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE;BUTTPARK 82\18-63;4-(Ethoxycarbonyl)-1-methyl-3-(trifluoromethyl)-1H-pyrazole;Ethyl 1-methyl-3-(trifluoromethyl)-1H-pyrazole-4-carboxylate ,97% | | CAS: | 111493-74-4 | | MF: | C8H9F3N2O2 | | MW: | 222.16 | | EINECS: | | | Product Categories: | Building Blocks;Pyrazole | | Mol File: | 111493-74-4.mol | 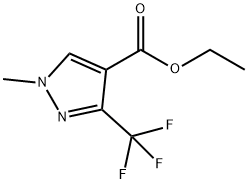 |
| | ETHYL 1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE Chemical Properties |
| Melting point | 60-61°C | | Boiling point | 267.4±40.0 °C(Predicted) | | density | 1.35±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | pka | -2.85±0.10(Predicted) | | form | solid | | Appearance | Light yellow to yellow Solid | | InChI | InChI=1S/C8H9F3N2O2/c1-3-15-7(14)5-4-13(2)12-6(5)8(9,10)11/h4H,3H2,1-2H3 | | InChIKey | ZZEXDJGNURSJOF-UHFFFAOYSA-N | | SMILES | N1(C)C=C(C(OCC)=O)C(C(F)(F)F)=N1 |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36/37/39 | | WGK Germany | WGK 3 | | HazardClass | IRRITANT | | HS Code | 29309090 | | Storage Class | 11 - Combustible Solids |
| | ETHYL 1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE Usage And Synthesis |
| Chemical Properties | White solid | | Synthesis | Ethyl 3-trifluoromethyl-1H-pyrazole-4-carboxylate (2.0 g, 9.61 mmol) was dissolved in N,N-dimethylformamide (20 mL) and the solution was cooled to 0 °C. Sodium hydride (0.58 g, 14.42 mmol, 60% dispersed in mineral oil) was slowly added to the solution at 0 °C and the reaction mixture was stirred for 20 min. Subsequently, iodomethane (0.90 mL, 14.42 mmol) was added and the reaction mixture was gradually warmed to room temperature over 18 hours. Upon completion of the reaction, the mixture was partitioned between ethyl acetate (100 mL) and water (50 mL). The organic layer was separated and washed sequentially with water (5 x 50 mL) and saturated aqueous sodium chloride solution (50 mL). The organic layer was dried with anhydrous sodium sulfate, filtered, concentrated and purified by silica gel column chromatography (eluent: 90:10 hexane/ethyl acetate) to afford ethyl 1-methyl-3-trifluoromethyl-1H-pyrazole-4-carboxylate (1.74 g, 81.7% yield).1H-NMR (400 MHz, CDCl3) δ 7.92 (s, 1H), 4.27 (q, 2H ), 3.95 (s, 3H), 1.33 (t, 3H). | | References | [1] Angewandte Chemie - International Edition, 2017, vol. 56, # 16, p. 4569 - 4574
[2] Angew. Chem., 2017, vol. 129, # 16, p. 4640 - 4645,6
[3] Patent: WO2008/141020, 2008, A1. Location in patent: Page/Page column 50
[4] Molecules, 2012, vol. 17, # 12, p. 14205 - 14218 |
| | ETHYL 1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE Preparation Products And Raw materials |
|