|
|
| | 2,4-Dichlorobenzothiazole Basic information |
| Product Name: | 2,4-Dichlorobenzothiazole | | Synonyms: | 2,4-Dichlorbenzothiazol;Benzothiazole, 2,4-dichloro-;2,4-Dichlorobenzo[d]thiazole;2,4-DICHLOROBENZOTHIAZOLE;2,4-DICHLORO-1,3-BENZOTHIAZOLE;IFLAB-BB F1910-0008;Benzothiazole, 2,4-dichloro- (7CI,8CI,9CI);sulfuric acid [[3-hydroxy-1-oxo-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-2-mercapto-2-oxanyl]pent-4-enyl]amino] ester | | CAS: | 3622-30-8 | | MF: | C7H3Cl2NS | | MW: | 204.08 | | EINECS: | 222-821-5 | | Product Categories: | BENZOTHIAZOLE | | Mol File: | 3622-30-8.mol | 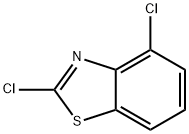 |
| | 2,4-Dichlorobenzothiazole Chemical Properties |
| Melting point | 96-97 °C | | Boiling point | 286.8±13.0 °C(Predicted) | | density | 1.567±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | pka | -2.02±0.10(Predicted) | | Appearance | Light yellow to orange Solid |
| | 2,4-Dichlorobenzothiazole Usage And Synthesis |
| Chemical Properties | Pale yellow solid | | Synthesis | Step B: 4-Chlorobenzo[d]thiazol-2-amine (4.78 g, 25.8 mmol) and copper(II) chloride (4.16 g, 31 mmol) were dissolved in acetonitrile (25 mL), followed by tert-butyl nitrite (4.61 mL, 38.8 mmol) at room temperature. The reaction mixture was heated to 60 °C and maintained for 30 min. Upon completion of the reaction, the solvent was removed by rotary evaporation. The residue was suspended in water and the solid product was collected by filtration and dried to give 2,4-dichlorobenzo[d]thiazole (5.3 g, 81% yield). Mass spectrometry analysis showed a m/z of 205.9 [M + H]+. | | References | [1] Chemistry - A European Journal, 2018, vol. 24, # 55, p. 14622 - 14626
[2] Patent: WO2013/101974, 2013, A1. Location in patent: Paragraph 00586; 00588
[3] Patent: US9617268, 2017, B2. Location in patent: Page/Page column 321; 322
[4] Gazzetta Chimica Italiana, 1964, vol. 94, p. 372 - 381
[5] Chemistry and Biodiversity, 2011, vol. 8, # 2, p. 253 - 265 |
| | 2,4-Dichlorobenzothiazole Preparation Products And Raw materials |
|