|
|
| | (R)-3-Amino-1-benzylpiperidine Basic information |
| Product Name: | (R)-3-Amino-1-benzylpiperidine | | Synonyms: | (R)-3-AMINO-1-BENZYL-PIPERIDINE;(R)-1-BENZYL-PIPERIDIN-3-YLAMINE;(R)-1-BENZYL-3-AMINOPIPERIDINE;1-BENZYL-PIPERIDIN-(R)-3-YLAMINE;C-3101R;1-Benzyl-3-aMinopiperidine 98% EE99%;(3R)-1-(Benzyl)-3-piperidinaMine;(r)-n-benzyl-3-amino-piperidine | | CAS: | 168466-84-0 | | MF: | C12H18N2 | | MW: | 190.28 | | EINECS: | | | Product Categories: | Piperidine | | Mol File: | 168466-84-0.mol | 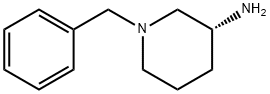 |
| | (R)-3-Amino-1-benzylpiperidine Chemical Properties |
| Boiling point | 281.2±33.0 °C(Predicted) | | density | 1.037±0.06 g/cm3(Predicted) | | Fp | 113℃ | | storage temp. | 2-8°C(protect from light) | | pka | 10.21±0.20(Predicted) | | form | solid | | color | Yellow | | Water Solubility | Slightly soluble in water. | | CAS DataBase Reference | 168466-84-0(CAS DataBase Reference) |
| | (R)-3-Amino-1-benzylpiperidine Usage And Synthesis |
| Chemical Properties | White powder or yellow liquid | | Uses | (R)-3-Amino-1-benzylpiperidine is used as an organic chemical synthesis intermediate. | | Synthesis | Example 33: Preparation of (R)-1-benzyl-3-aminopiperidine
(R)-1-benzylpiperidinamide (1.1 g) described in Example 2 was used as a starting material, which was mixed with water (5 mL), 30% aqueous sodium hydroxide (6.7 g, 10 eq.), tetrahydrofuran (THF, 10 mL), and an aqueous sodium hypochlorite solution (11.2 g, 1.5 eq.). The reaction mixture was stirred at 15°C for 1 hour, then warmed to 60°C and continued stirring for 2 hours. Upon completion of the reaction, the mixture was cooled to 20 °C and extracted three times with toluene (10 mL). After combining the organic layers, the solvent was removed by concentration under reduced pressure to afford the target product (R)-3-amino-1-benzylpiperidine as a yellow-brown oil (0.8 g, yield: 55%). | | References | [1] Patent: US2010/105917, 2010, A1. Location in patent: Page/Page column 21 |
| | (R)-3-Amino-1-benzylpiperidine Preparation Products And Raw materials |
|