- N-CYANOACETYLURETHANE
-

- $0.00 / 25kg
-
2026-01-05
- CAS:6629-04-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- N-Cyanoacetylurethane
-

- $0.00 / 25kg
-
2025-11-19
- CAS:6629-04-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- N-cyanoacetylurethane
-

- $0.00 / 1kg
-
2024-11-08
- CAS:6629-04-5
- Min. Order: 1kg
- Purity: 99%min
- Supply Ability: 1 tons
|
| | N-CYANOACETYLURETHANE Basic information |
| Product Name: | N-CYANOACETYLURETHANE | | Synonyms: | N-Cyanoacetylurethane 98%;Ethyl (2-cyanoacetyl);2-Cyanoacetylurethane;Carbamic acid, (cyanoacetyl)-, ethyl ester;cyanoacetyl-carbamicaciethylester;Cyanoacetylurethan;Ethyl cyanacetylcarbamate;N-Cyanoacetyl ethyl carbamate | | CAS: | 6629-04-5 | | MF: | C6H8N2O3 | | MW: | 156.14 | | EINECS: | 229-615-4 | | Product Categories: | intermediate;1 | | Mol File: | 6629-04-5.mol | 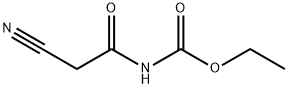 |
| | N-CYANOACETYLURETHANE Chemical Properties |
| Melting point | 167-169 °C (lit.) | | Boiling point | 280.35°C (rough estimate) | | density | 1.197 | | refractive index | 1.5010 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO, Methanol | | form | Solid | | pka | 3.46±0.10(Predicted) | | color | Pale Yellow | | InChI | InChI=1S/C6H8N2O3/c1-2-11-6(10)8-5(9)3-4-7/h2-3H2,1H3,(H,8,9,10) | | InChIKey | HSOGVWWWGVFXGF-UHFFFAOYSA-N | | SMILES | C(OCC)(=O)NC(CC#N)=O |
| Hazard Codes | Xn | | Risk Statements | 20/21/22-36/37/38 | | Safety Statements | 26-37/39 | | WGK Germany | 3 | | RTECS | EZ3480000 | | HazardClass | IRRITANT | | HS Code | 2926907090 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Acute Tox. 4 Dermal
Acute Tox. 4 Inhalation
Acute Tox. 4 Oral
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| | N-CYANOACETYLURETHANE Usage And Synthesis |
| Chemical Properties | Pale Yellow Solid | | Uses | N-Cyanoacetylurethane is used in the synthesis of inhibitors targetting the PDZ domain of PICK1, which is a potential target for brain ischemia, pain and addiction illnesses. Also it is used in the synthesis of cathespin K inhibitors. | | Reactions |
N-Cyanoacetylurethane has multiple reactivity and can participate in diazotization reactions and coordinate reactions with metal complexes.
| | Synthesis | 2-Cyanoacetic acid was reacted with ethyl carbamate, DMF and phosphorus oxychloride, and finally extracted with ethyl acetate to obtain N-Cyanoacetylurethane.
| | References | [1] Patent: WO2014/190199, 2014, A1. Location in patent: Paragraph 00250
[2] Patent: WO2016/81522, 2016, A1. Location in patent: Paragraph 00284; 00285; 00290
[3] Organic and Biomolecular Chemistry, 2010, vol. 8, # 19, p. 4281 - 4288
[4] Patent: US2013/252958, 2013, A1. Location in patent: Paragraph 0094
[5] Angewandte Chemie - International Edition, 2017, vol. 56, # 42, p. 13011 - 13015 |
| | N-CYANOACETYLURETHANE Preparation Products And Raw materials |
|